Exploring Guolin Qigong (Mind-Body Exercise) for Improving Cancer Related Fatigue in Cancer Survivors: A Mixed Method Randomized Controlled Trial Protocol
- PMID: 38757745
- PMCID: PMC11102686
- DOI: 10.1177/15347354241252698
Exploring Guolin Qigong (Mind-Body Exercise) for Improving Cancer Related Fatigue in Cancer Survivors: A Mixed Method Randomized Controlled Trial Protocol
Abstract
Background: Cancer-related fatigue and its associated symptoms of sleep disorder and depression are prevalent in cancer survivors especially among breast, lung, and colorectal cancer survivors. While there is no gold standard for treating cancer-related fatigue currently, studies of mind-body exercises such as Qigong have reported promise in reducing symptoms. This study was designed to evaluate the feasibility and effect of Guolin Qigong on cancer-related fatigue and other symptoms in breast, lung and colorectal cancer survivors while exploring their perceptions and experiences of Guolin Qigong intervention.
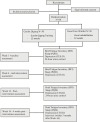
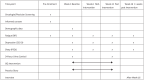
Methods: This is an open-label randomized controlled trial with 60 participants divided into 2 study groups in a 1:1 ratio. The intervention group will receive 12 weeks of Guolin Qigong intervention with a 4-week follow-up while control will receive usual care under waitlist. The primary outcome will be feasibility measured based on recruitment and retention rates, class attendance, home practice adherence, nature, and quantum of missing data as well as safety. The secondary subjective outcomes of fatigue, sleep quality and depression will be measured at Week-1 (baseline), Week-6 (mid-intervention), Week-12 (post-intervention), and Week-16 (4 weeks post-intervention) while an objective 24-hour urine cortisol will be measured at Week-1 (baseline) and Week-12 (post-intervention). We will conduct a semi-structured interview individually with participants within 3 months after Week-16 (4 weeks post-intervention) to obtain a more comprehensive view of practice adherence.
Discussion: This is the first mixed-method study to investigate the feasibility and effect of Guolin Qigong on breast, lung, and colorectal cancer survivors to provide a comprehensive understanding of Guolin Qigong's intervention impact and participants' perspectives. The interdisciplinary collaboration between Western Medicine and Chinese Medicine expertise of this study ensures robust study design, enhanced participant care, rigorous data analysis, and meaningful interpretation of results. This innovative research contributes to the field of oncology and may guide future evidence-based mind-body interventions to improve cancer survivorship.
Trial registration: This study has been registered with ANZCTR (ACTRN12622000688785p), was approved by Medical Research Ethic Committee of University Malaya Medical Centre (MREC ID NO: 2022323-11092) and recognized by Western Sydney University Human Research Ethics Committee (RH15124).
Keywords: Qigong; cancer; cancer survivors; cortisol; depression; fatigue; insomnia; mind body exercise; sleep.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
References
-
- Peters ME, Goedendorp MM, Verhagen CA, van der Graaf WT, Bleijenberg G. Severe fatigue during the palliative treatment phase of cancer: an exploratory study. Cancer Nurs. 2014;37:139-145. - PubMed
-
- Jones JM, Olson K, Catton P, et al. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J Cancer Surviv. 2016;10:51-61. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical