Applying improved ddPCR to reliable quantification of MPXV in clinical settings
- PMID: 38757960
- PMCID: PMC11218477
- DOI: 10.1128/spectrum.00018-24
Applying improved ddPCR to reliable quantification of MPXV in clinical settings
Abstract
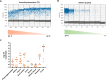
Monkeypox virus (MPXV) poses a global health threat. Droplet digital PCR (ddPCR) holds potential as an accurate diagnostic tool for clinical microbiology. However, there is limited literature on the applicability of ddPCR in clinical settings. In this study, the clinical features of patients with MPXV during the initial outbreak in China in June 2023 were reviewed, and an optimized ddPCR method with dilution and/or inhibitor removal was developed to enhance MPXV detection efficiency. Eighty-two MPXV samples were tested from nine different clinical specimen types, including feces, urine, pharyngeal swabs, anal swabs, saliva, herpes fluid, crust, and semen, and the viral load of each specimen was quantified. A comparative analysis was performed with qPCR to assess sensitivity and specificity and to investigate the characteristics of MPXV infection by analyzing viral loads in different clinical specimens. Consequently, common pharyngeal and gastrointestinal symptoms were observed in patients with MPXV. The optimized ddPCR method demonstrated relatively high sensitivity for MPXV quantification in the clinical materials, with a limit of detection of 0.1 copies/μL. This was particularly evident in low-concentration samples like whole blood, semen, and urine. The optimized ddPCR demonstrated greater detection accuracy compared with normal ddPCR and qPCR, with an area under the curve (AUC) of 0.939. Except for crust samples, viral loads in the specimens gradually decreased as the disease progressed. Virus levels in feces and anal swabs kept a high detection rate at each stage of post-symptom onset, and feces and anal swabs samples may be suitable for clinical diagnosis and continuous monitoring of MPXV.
Importance: The ddPCR technique proved to be a sensitive and valuable tool for accurately quantifying MPXV viral loads in various clinical specimen types. The findings provided valuable insights into the necessary pre-treatment protocols for MPXV diagnosis in ddPCR detection and the potentially suitable sample types for collection. Therefore, such results can aid in comprehending the potential characteristics of MPXV infection and the usage of ddPCR in clinical settings.
Keywords: Mpox; ddPCR; monkeypox virus; quantification; viral loads.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Alternative sampling specimens for the molecular detection of mpox (formerly monkeypox) virus.J Clin Virol. 2023 Feb;159:105372. doi: 10.1016/j.jcv.2022.105372. Epub 2022 Dec 26. J Clin Virol. 2023. PMID: 36608620 Free PMC article.
-
Clinical characteristics, viral dynamics, and antibody response of monkeypox virus infections among men with and without HIV infection in Guangzhou, China.Front Cell Infect Microbiol. 2024 Jun 24;14:1412753. doi: 10.3389/fcimb.2024.1412753. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38979508 Free PMC article.
-
Evaluation and clinical validation of monkeypox (mpox) virus real-time PCR assays.J Clin Virol. 2023 Feb;159:105373. doi: 10.1016/j.jcv.2022.105373. Epub 2022 Dec 23. J Clin Virol. 2023. PMID: 36603329 Free PMC article.
-
Monkeypox outbreak: Wastewater and environmental surveillance perspective.Sci Total Environ. 2023 Jan 15;856(Pt 2):159166. doi: 10.1016/j.scitotenv.2022.159166. Epub 2022 Oct 3. Sci Total Environ. 2023. PMID: 36202364 Free PMC article. Review.
-
Monkeypox viral detection in semen specimens of confirmed cases: A systematic review and meta-analysis.J Med Virol. 2023 Jan;95(1):e28250. doi: 10.1002/jmv.28250. Epub 2022 Nov 3. J Med Virol. 2023. PMID: 36271741
Cited by
-
A Comparative Evaluation of Three Diagnostic Assays for the Detection of Human Monkeypox.Viruses. 2024 Aug 12;16(8):1286. doi: 10.3390/v16081286. Viruses. 2024. PMID: 39205260 Free PMC article.
-
Mpox caused by Clade Ib: Epidemiological characteristics, prevention, and control.Chin Med J (Engl). 2025 Jan 23;138(5):505-8. doi: 10.1097/CM9.0000000000003464. Online ahead of print. Chin Med J (Engl). 2025. PMID: 39844015 Free PMC article. No abstract available.
References
-
- World Health Organization . 2023. 2022-23 Mpox (monkeypox) outbreak: global trends. Available from: https://worldhealthorg.shinyapps.io/mpx_global/#1_Overview. Retrieved 25 Dec 2023.
-
- O’Toole Á, Neher RA, Ndodo N, Borges V, Gannon B, Gomes JP, Groves N, King DJ, Maloney D, Lemey P, Lewandowski K, Loman N, Myers R, Omah IF, Suchard MA, Worobey M, Chand M, Ihekweazu C, Ulaeto D, Adetifa I, Rambaut A. 2023. APOBEC3 deaminase editing in mpox virus as evidence for sustained human transmission since at least 2016. Science 382:595–600. doi:10.1126/science.adg8116 - DOI - PMC - PubMed
-
- Ogoina D, Izibewule JH, Ogunleye A, Ederiane E, Anebonam U, Neni A, Oyeyemi A, Etebu EN, Ihekweazu C. 2019. The 2017 human monkeypox outbreak in Nigeria-Report of outbreak experience and response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS One 14:e0214229. doi:10.1371/journal.pone.0214229 - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

