Temperate phage-antibiotic synergy across antibiotic classes reveals new mechanism for preventing lysogeny
- PMID: 38757974
- PMCID: PMC11237771
- DOI: 10.1128/mbio.00504-24
Temperate phage-antibiotic synergy across antibiotic classes reveals new mechanism for preventing lysogeny
Abstract
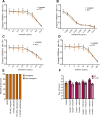
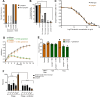
A recent demonstration of synergy between a temperate phage and the antibiotic ciprofloxacin suggested a scalable approach to exploiting temperate phages in therapy, termed temperate phage-antibiotic synergy, which specifically interacted with the lysis-lysogeny decision. To determine whether this would hold true across antibiotics, we challenged Escherichia coli with the phage HK97 and a set of 13 antibiotics spanning seven classes. As expected, given the conserved induction pathway, we observed synergy with classes of drugs known to induce an SOS response: a sulfa drug, other quinolones, and mitomycin C. While some β-lactams exhibited synergy, this appeared to be traditional phage-antibiotic synergy, with no effect on the lysis-lysogeny decision. Curiously, we observed a potent synergy with antibiotics not known to induce the SOS response: protein synthesis inhibitors gentamicin, kanamycin, tetracycline, and azithromycin. The synergy results in an eightfold reduction in the effective minimum inhibitory concentration of gentamicin, complete eradication of the bacteria, and, when administered at sub-optimal doses, drastically decreases the frequency of lysogens emerging from the combined challenge. However, lysogens exhibit no increased sensitivity to the antibiotic; synergy was maintained in the absence of RecA; and the antibiotic reduced the initial frequency of lysogeny rather than selecting against formed lysogens. Our results confirm that SOS-inducing antibiotics broadly result in temperate-phage-specific synergy, but that other antibiotics can interact with temperate phages specifically and result in synergy. This is the first report of a means of chemically blocking entry into lysogeny, providing a new means for manipulating the key lysis-lysogeny decision.IMPORTANCEThe lysis-lysogeny decision is made by most bacterial viruses (bacteriophages, phages), determining whether to kill their host or go dormant within it. With over half of the bacteria containing phages waiting to wake, this is one of the most important behaviors in all of biology. These phages are also considered unusable for therapy because of this behavior. In this paper, we show that many antibiotics bias this behavior to "wake" the dormant phages, forcing them to kill their host, but some also prevent dormancy in the first place. These will be important tools to study this critical decision point and may enable the therapeutic use of these phages.
Keywords: antimicrobial resistance; bacteriophage; lysis-lysogeny; phage-antibiotic synergy; temperate phage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Temperate phage-antibiotic synergy is widespread-extending to Pseudomonas-but varies by phage, host strain, and antibiotic pairing.mBio. 2025 Feb 5;16(2):e0255924. doi: 10.1128/mbio.02559-24. Epub 2024 Dec 20. mBio. 2025. PMID: 39704503 Free PMC article.
-
Temperate phage-antibiotic synergy eradicates bacteria through depletion of lysogens.Cell Rep. 2021 May 25;35(8):109172. doi: 10.1016/j.celrep.2021.109172. Cell Rep. 2021. PMID: 34038739
-
Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry.mBio. 2020 Aug 4;11(4):e01462-20. doi: 10.1128/mBio.01462-20. mBio. 2020. PMID: 32753497 Free PMC article.
-
Molecular Basis of Lysis-Lysogeny Decisions in Gram-Positive Phages.Annu Rev Microbiol. 2021 Oct 8;75:563-581. doi: 10.1146/annurev-micro-033121-020757. Epub 2021 Aug 3. Annu Rev Microbiol. 2021. PMID: 34343015 Review.
-
Bacteriophages and antibiotic interactions in clinical practice: what we have learned so far.J Biomed Sci. 2022 Mar 30;29(1):23. doi: 10.1186/s12929-022-00806-1. J Biomed Sci. 2022. PMID: 35354477 Free PMC article. Review.
Cited by
-
Re-evaluating what makes a phage unsuitable for therapy.NPJ Antimicrob Resist. 2025 May 29;3(1):45. doi: 10.1038/s44259-025-00117-z. NPJ Antimicrob Resist. 2025. PMID: 40442282 Free PMC article. Review.
-
Genomic and Functional Characterization of Novel Phages Targeting Multidrug-Resistant Acinetobacter baumannii.Int J Mol Sci. 2025 Jun 26;26(13):6141. doi: 10.3390/ijms26136141. Int J Mol Sci. 2025. PMID: 40649915 Free PMC article.
-
Antagonistic effect of kanamycin on kanamycin-resistant Escherichia coli infection by BtuB-targeting bacteriophages.Arch Virol. 2025 Jul 3;170(8):172. doi: 10.1007/s00705-025-06358-7. Arch Virol. 2025. PMID: 40603587
-
Characterization and Comparative Genomic Analysis of vB_BceM_CEP1: A Novel Temperate Bacteriophage Infecting Burkholderia cepacia Complex.J Microbiol. 2024 Nov;62(11):1035-1055. doi: 10.1007/s12275-024-00185-2. Epub 2024 Nov 18. J Microbiol. 2024. PMID: 39557803
-
Temperate phage-antibiotic synergy is widespread-extending to Pseudomonas-but varies by phage, host strain, and antibiotic pairing.mBio. 2025 Feb 5;16(2):e0255924. doi: 10.1128/mbio.02559-24. Epub 2024 Dec 20. mBio. 2025. PMID: 39704503 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
