Comparison of the performance of two targeted metagenomic virus capture probe-based methods using reference control materials and clinical samples
- PMID: 38757981
- PMCID: PMC11237577
- DOI: 10.1128/jcm.00345-24
Comparison of the performance of two targeted metagenomic virus capture probe-based methods using reference control materials and clinical samples
Abstract
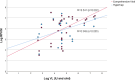
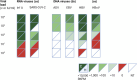
Viral enrichment by probe hybridization has been reported to significantly increase the sensitivity of viral metagenomics. This study compares the analytical performance of two targeted metagenomic virus capture probe-based methods: (i) SeqCap EZ HyperCap by Roche (ViroCap) and (ii) Twist Comprehensive Viral Research Panel workflow, for diagnostic use. Sensitivity, specificity, and limit of detection were analyzed using 25 synthetic viral sequences spiked in increasing proportions of human background DNA, eight clinical samples, and American Type Culture Collection (ATCC) Virome Virus Mix. Sensitivity and specificity were 95% and higher for both methods using the synthetic and reference controls as gold standard. Combining thresholds for viral sequence read counts and genome coverage [respectively 500 reads per million (RPM) and 10% coverage] resulted in optimal prediction of true positive results. Limits of detection were approximately 50-500 copies/mL for both methods as determined by ddPCR. Increasing proportions of spike-in cell-free human background sequences up to 99.999% (50 ng/mL) did not negatively affect viral detection, suggesting effective capture of viral sequences. These data show analytical performances in ranges applicable to clinical samples, for both probe hybridization metagenomic approaches. This study supports further steps toward more widespread use of viral metagenomics for pathogen detection, in clinical and surveillance settings using low biomass samples.
Importance: Viral metagenomics has been gradually applied for broad-spectrum pathogen detection of infectious diseases, surveillance of emerging diseases, and pathogen discovery. Viral enrichment by probe hybridization methods has been reported to significantly increase the sensitivity of viral metagenomics. During the past years, a specific hybridization panel distributed by Roche has been adopted in a broad range of different clinical and zoonotic settings. Recently, Twist Bioscience has released a new hybridization panel targeting human and animal viruses. This is the first report comparing the performance of viral metagenomic hybridization panels.
Keywords: capture probes; targeted metagenomics; viral diagnostics; viral metagenomics.
Conflict of interest statement
D.V.D.M. and A.B. are employees of GenomeScan B.V. and provided the sequencing service. They were not involved in bioinformatic/statistical data analysis or interpretation of results.
Figures



Similar articles
-
Detection of Viruses in Clinical Samples by Use of Metagenomic Sequencing and Targeted Sequence Capture.J Clin Microbiol. 2018 Nov 27;56(12):e01123-18. doi: 10.1128/JCM.01123-18. Print 2018 Dec. J Clin Microbiol. 2018. PMID: 30232133 Free PMC article.
-
Evaluating metagenomics and targeted approaches for diagnosis and surveillance of viruses.Genome Med. 2024 Sep 9;16(1):111. doi: 10.1186/s13073-024-01380-x. Genome Med. 2024. PMID: 39252069 Free PMC article.
-
Multicenter benchmarking of short and long read wet lab protocols for clinical viral metagenomics.J Clin Virol. 2024 Aug;173:105695. doi: 10.1016/j.jcv.2024.105695. Epub 2024 May 25. J Clin Virol. 2024. PMID: 38823290
-
Metagenomics and future perspectives in virus discovery.Curr Opin Virol. 2012 Feb;2(1):63-77. doi: 10.1016/j.coviro.2011.12.004. Epub 2012 Jan 20. Curr Opin Virol. 2012. PMID: 22440968 Free PMC article. Review.
-
The Human Virome: Viral Metagenomics, Relations with Human Diseases, and Therapeutic Applications.Viruses. 2022 Jan 28;14(2):278. doi: 10.3390/v14020278. Viruses. 2022. PMID: 35215871 Free PMC article. Review.
Cited by
-
The 2023 South Sudanese outbreak of Hepatitis E emphasizes ongoing circulation of genotype 1 in North, Central, and East Africa.Infect Genet Evol. 2024 Oct;124:105667. doi: 10.1016/j.meegid.2024.105667. Epub 2024 Sep 7. Infect Genet Evol. 2024. PMID: 39251076 Free PMC article.
-
Metagenomic and transcriptomic investigation of pediatric acute liver failure cases reveals a common pathway predominated by monocytes.mBio. 2025 Apr 9;16(4):e0391324. doi: 10.1128/mbio.03913-24. Epub 2025 Mar 18. mBio. 2025. PMID: 40099881 Free PMC article.
-
Evaluation of the Impact of Concentration and Extraction Methods on the Targeted Sequencing of Human Viruses from Wastewater.Environ Sci Technol. 2024 May 14;58(19):8239-8250. doi: 10.1021/acs.est.4c00580. Epub 2024 May 1. Environ Sci Technol. 2024. PMID: 38690747 Free PMC article.
-
Hybrid Capture-Based Sequencing Enables Highly Sensitive Zoonotic Virus Detection Within the One Health Framework.Pathogens. 2025 Mar 7;14(3):264. doi: 10.3390/pathogens14030264. Pathogens. 2025. PMID: 40137749 Free PMC article.
-
Air sampling accurately captures circulating zoonotic viral diversity emerging from poultry live-animal markets.Res Sq [Preprint]. 2025 Feb 13:rs.3.rs-5682962. doi: 10.21203/rs.3.rs-5682962/v1. Res Sq. 2025. PMID: 39989955 Free PMC article. Preprint.
References
-
- Deng X, Achari A, Federman S, Yu G, Somasekar S, Bártolo I, Yagi S, Mbala-Kingebeni P, Kapetshi J, Ahuka-Mundeke S, et al. . 2020. Metagenomic sequencing with spiked primer enrichment for viral diagnostics and genomic surveillance. Nat Microbiol 5:443–454. doi:10.1038/s41564-019-0637-9 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

