Classical swine fever virus non-structural protein 4A recruits dihydroorotate dehydrogenase to facilitate viral replication
- PMID: 38757985
- PMCID: PMC11237749
- DOI: 10.1128/jvi.00494-24
Classical swine fever virus non-structural protein 4A recruits dihydroorotate dehydrogenase to facilitate viral replication
Abstract
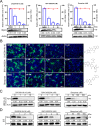
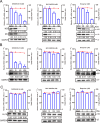
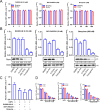
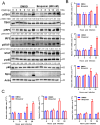
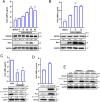
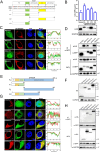
Mitochondria are energy producers in cells, which can affect viral replication by regulating the host innate immune signaling pathways, and the changes in their biological functions are inextricably linked the viral life cycle. In this study, we screened a library of 382 mitochondria-targeted compounds and identified the antiviral inhibitors of dihydroorotate dehydrogenase (DHODH), the rate-limiting enzyme in the de novo synthesis pathway of pyrimidine ribonucleotides, against classical swine fever virus (CSFV). Our data showed that the inhibitors interfered with viral RNA synthesis in a dose-dependent manner, with half-maximal effective concentrations (EC50) ranging from 0.975 to 26.635 nM. Remarkably, DHODH inhibitors obstructed CSFV replication by enhancing the innate immune response including the TBK1-IRF3-STAT1 and NF-κB signaling pathways. Furthermore, the data from a series of compound addition and supplementation trials indicated that DHODH inhibitors also inhibited CSFV replication by blocking the de novo pyrimidine synthesis. Remarkably, DHODH knockdown demonstrated that it was essential for CSFV replication. Mechanistically, confocal microscopy and immunoprecipitation assays showed that the non-structural protein 4A (NS4A) recruited and interacted with DHODH in the perinuclear. Notably, NS4A enhanced the DHODH activity and promoted the generation of UMP for efficient viral replication. Structurally, the amino acids 65-229 of DHODH and the amino acids 25-40 of NS4A were pivotal for this interaction. Taken together, our findings highlight the critical role of DHODH in the CSFV life cycle and offer a potential antiviral target for the development of novel therapeutics against CSF.
Importance: Classical swine fever remains one of the most economically important viral diseases of domestic pigs and wild boar worldwide. dihydroorotate dehydrogenase (DHODH) inhibitors have been shown to suppress the replication of several viruses in vitro and in vivo, but the effects on Pestivirus remain unknown. In this study, three specific DHODH inhibitors, including DHODH-IN-16, BAY-2402234, and Brequinar were found to strongly suppress classical swine fever virus (CSFV) replication. These inhibitors target the host DHODH, depleting the pyrimidine nucleotide pool to exert their antiviral effects. Intriguingly, we observed that the non-structural protein 4A of CSFV induced DHODH to accumulate around the nucleus in conjunction with mitochondria. Moreover, NS4A exhibited a strong interaction with DHODH, enhancing its activity to promote efficient CSFV replication. In conclusion, our findings enhance the understanding of the pyrimidine synthesis in CSFV infection and expand the novel functions of CSFV NS4A in viral replication, providing a reference for further exploration of antiviral targets against CSFV.
Keywords: DHODH; classical swine fever virus (CSFV); interaction; non-structural protein 4A (NS4A); pyrimidine; viral replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Liu C-C, Liu Y-Y, Zhou J-F, Chen X, Chen H, Hu J-H, Chen J, Zhang J, Sun R-C, Wei J-C, Go YY, Morita E, Zhou B. 2022. Cellular ESCRT components are recruited to regulate the endocytic trafficking and RNA replication compartment assembly during classical swine fever virus infection. PLoS Pathog 18:e1010294. doi: 10.1371/journal.ppat.1010294 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

