Timing-dependent synergies between motor cortex and posterior spinal stimulation in humans
- PMID: 38758005
- PMCID: PMC11178459
- DOI: 10.1113/JP286183
Timing-dependent synergies between motor cortex and posterior spinal stimulation in humans
Abstract
Volitional movement requires descending input from the motor cortex and sensory feedback through the spinal cord. We previously developed a paired brain and spinal electrical stimulation approach in rats that relies on convergence of the descending motor and spinal sensory stimuli in the cervical cord. This approach strengthened sensorimotor circuits and improved volitional movement through associative plasticity. In humans, it is not known whether posterior epidural spinal cord stimulation targeted at the sensorimotor interface or anterior epidural spinal cord stimulation targeted within the motor system is effective at facilitating brain evoked responses. In 59 individuals undergoing elective cervical spine decompression surgery, the motor cortex was stimulated with scalp electrodes and the spinal cord was stimulated with epidural electrodes, with muscle responses being recorded in arm and leg muscles. Spinal electrodes were placed either posteriorly or anteriorly, and the interval between cortex and spinal cord stimulation was varied. Pairing stimulation between the motor cortex and spinal sensory (posterior) but not spinal motor (anterior) stimulation produced motor evoked potentials that were over five times larger than brain stimulation alone. This strong augmentation occurred only when descending motor and spinal afferent stimuli were timed to converge in the spinal cord. Paired stimulation also increased the selectivity of muscle responses relative to unpaired brain or spinal cord stimulation. Finally, clinical signs suggest that facilitation was observed in both injured and uninjured segments of the spinal cord. The large effect size of this paired stimulation makes it a promising candidate for therapeutic neuromodulation. KEY POINTS: Pairs of stimuli designed to alter nervous system function typically target the motor system, or one targets the sensory system and the other targets the motor system for convergence in cortex. In humans undergoing clinically indicated surgery, we tested paired brain and spinal cord stimulation that we developed in rats aiming to target sensorimotor convergence in the cervical cord. Arm and hand muscle responses to paired sensorimotor stimulation were more than five times larger than brain or spinal cord stimulation alone when applied to the posterior but not anterior spinal cord. Arm and hand muscle responses to paired stimulation were more selective for targeted muscles than the brain- or spinal-only conditions, especially at latencies that produced the strongest effects of paired stimulation. Measures of clinical evidence of compression were only weakly related to the paired stimulation effect, suggesting that it could be applied as therapy in people affected by disorders of the central nervous system.
Keywords: cervical; electrical stimulation; epidural; motor cortex; motor evoked potentials; myelopathy; spinal cord.
© 2024 The Authors. The Journal of Physiology © 2024 The Physiological Society.
Conflict of interest statement
5 Additional Information
5.1 Competing interests
Figures
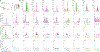



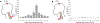
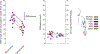

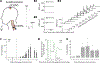
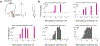

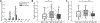

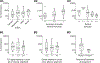
Update of
-
Timing dependent synergies between motor cortex and posterior spinal stimulation in humans.medRxiv [Preprint]. 2023 Dec 21:2023.08.18.23294259. doi: 10.1101/2023.08.18.23294259. medRxiv. 2023. Update in: J Physiol. 2024 Jun;602(12):2961-2983. doi: 10.1113/JP286183. PMID: 37645795 Free PMC article. Updated. Preprint.
References
-
- Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, Ferreira CK & Harkema SJ (2018). Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N Engl J Med 379, 1244–1250. - PubMed
-
- Barzilai O, Laufer I & Bilsky MH (2019). Posterolateral Approach to Thoraco-Lumbar Metastases - Separation Surgery. In Spinal Tumor Surgery: A Case-Based Approach, ed. Sciubba DM, pp. 177–184. Springer International Publishing, Cham.
-
- Benzel EC, Lancon J, Kesterson L & Hadden T (1991). Cervical Laminectomy and Dentate Ligament Section for Cervical Spondylotic Myelopathy. Clin Spine Surg 4, 286. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

