Effect of HLA Genotype on Anti-PD-1 Antibody Treatment for Advanced Renal Cell Carcinoma in the SNiP-RCC Study
- PMID: 38758119
- PMCID: PMC11212726
- DOI: 10.4049/jimmunol.2300308
Effect of HLA Genotype on Anti-PD-1 Antibody Treatment for Advanced Renal Cell Carcinoma in the SNiP-RCC Study
Abstract
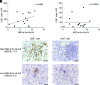
Immune checkpoint blockade therapies are widely used for cancer treatment, including advanced renal cell carcinoma (RCC). This study aimed to investigate the impact of zygosity in HLA genes and individual HLA genotypes on the efficacy of an anti-PD-1 Ab, nivolumab, in treating advanced RCC. Patient enrollment was conducted across 23 institutions in Japan from August 19, 2019, to September 30, 2020, with follow-up concluding on March 31, 2021. HLA genotype imputation of HLA-A, B, and C, DQB1, and DRB1 loci was performed. Among 222 patients, the presence of at least one homozygosity of the HLA-II allele significantly improved the best objective response (hazard ratio, 0.34; 95% confidence interval, 0.21-0.96; p = 0.042). The HLA evolutionary divergence (HED) of the HLA-A and HLA-B loci was higher than the HLA-C (p < 0.0001 and p < 0.0001, respectively), with high HED of the HLA-B locus correlating to clinical benefits in nivolumab treatment (hazard ratio, 0.44; 95% confidence interval, 0.21-0.90; p = 0.024) and improving cancer-specific survival compared with the low group (p = 0.0202). Additionally, high HED of the HLA-B locus was correlated with the number of infiltrated CD8+ cells in the tumor microenvironment (correlation coefficient, 0.4042). These findings indicate that the diversity of the HLA-B locus plays a significant role in the anti-tumor effect of nivolumab treatment in advanced RCC, potentially offering insights for improved risk stratification in nivolumab treatment and leading to better medical management of advanced RCC.
Copyright © 2024 by The American Association of Immunologists, Inc.
Conflict of interest statement
H.M. received honoraria from Ono Pharmaceutical. M.T. received honoraria from Ono Pharmaceutical and Bristol-Myers Squibb. M.O. received honoraria from Pfizer, Novartis, Bayer, MSD, Eisai, Ono Pharmaceutical, Bristol-Myers Squibb, Takeda Pharmaceutical and Merck Biopharma. N.T. received honoraria from Pfizer, Novartis, MSD, Eisai, Bristol-Myers Squibb, Takeda Pharmaceutical, and Merck Biopharma and support for attending meetings from Merck Biopharma. N.M. received honoraria from MSD, Bristol-Myers Squibb, Takeda Pharmaceutical, and Ono Pharmaceutical. H.M. received grants from Janssen Pharma, Takeda Pharmaceutical, Baxter, KyowaKirin, and Sanofi and honoraria from Janssen Pharma, AstraZeneca, Merck Biopharma, Bayer, Astellas Pharma, and Chugai. W.O. received honoraria from Ono Pharmaceutical, Merck Biopharma, Takeda Pharmaceutical, and Astellas Pharma. N.S. received grants from Ono Pharmaceutical, honoraria from Ono Pharmaceutical and Bristol-Myers Squibb, and support for attending meetings from Ono Pharmaceutical and Bristol-Myers Squibb. M.N. received honoraria from Takeda Pharmaceutical. S.A. received grants from Astellas Pharma, AstraZeneca, and Tosoh and honoraria from Janssen Pharma, AstraZeneca, Bayer, Astellas Pharma, Sanofi, and Takeda Pharmaceutical. T.K. received honoraria from AstraZeneca and Merck Biopharma. M.E. received grants from Ono Pharmaceutical and honoraria from Ono Pharmaceutical and Bristol-Myers Squibb. All other authors have no financial conflicts of interest.
Figures


References
-
- Motzer, R. J., Rini B. I., McDermott D. F., Arén Frontera O., Hammers H. J., Carducci M. A., Salman P., Escudier B., Beuselinck B., Amin A., et al. . CheckMate 214 investigators . 2019. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 20: 1370–1385. - PMC - PubMed
-
- Rini, B. I., Plimack E. R., Stus V., Gafanov R., Hawkins R., Nosov D., Pouliot F., Alekseev B., Soulières D., Melichar B., et al. . KEYNOTE-426 Investigators . 2019. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 380: 1116–1127. - PubMed
-
- Choueiri, T. K., Powles T., Burotto M., Escudier B., Bourlon M. T., Zurawski B., Oyervides Juárez V. M., Hsieh J. J., Basso U., Shah A. Y., et al. . CheckMate 9ER Investigators . 2021. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 384: 829–841. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

