The lymphocyte/monocyte ratio predicts the efficacy of isatuximab plus pomalidomide in multiple myeloma patients
- PMID: 38758239
- PMCID: PMC11101389
- DOI: 10.1007/s00262-024-03711-8
The lymphocyte/monocyte ratio predicts the efficacy of isatuximab plus pomalidomide in multiple myeloma patients
Abstract
Background: Isatuximab, an anti-CD38 antibody, has been widely used in treatments for patients with relapsed/refractory multiple myeloma (MM). Despite its high efficacy, not all patients achieve a lasting therapeutic response with isatuximab.
Objective: We tried to identify biomarkers to predict the effectiveness of isatuximab by focusing on the host's immune status before treatment.
Methods: We retrospectively analyzed the cases of 134 relapsed/refractory MM patients in the Kansai Myeloma Forum database who had received only a first isatuximab treatment.
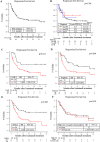
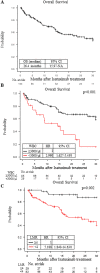
Results: Among the 134 patients, an isatuximab, pomalidomide and dexamethasone (Isa-PD) regimen, isatuximab, carfilzomib and dexamethasone (Isa-KD) regimen and isatuximab and/or dexamethasone (Isa-D) regimen were used in 112, 15 and 7 patients, respectively. The median age at treatment, number of prior treatment regimens, and progression-free survival (PFS) were 71, 6, and 6.54 months, respectively. Multivariate analysis showed that the PFS under the Isa-PD regimen was longer in patients with higher lymphocyte/monocyte ratio (LMR ≥ 4), fewer prior treatment regimens (< 6), and no use of prior daratumumab treatment. The OS under the Isa-PD regimen was longer in patients with higher white blood cell counts (WBC counts ≥ 3000/μL) and higher LMR. The PFS under the Isa-D regimen was longer in patients with fewer prior treatment regimens in univariate analysis, but no parameters were correlated with PFS/OS under the Isa-KD regimen.
Conclusion: We found that the patients with higher LMR (≥ 4) could obtain longer PFS and OS under the Isa-PD regimen. Other cohort studies of isatuximab treatment might be necessary to substantiate our results.
Keywords: Isatuximab; Lymphocyte/monocyte ratio; Multiple myeloma; Predictive markers.
© 2024. The Author(s).
Conflict of interest statement
Shin-ichi Fuchida has received honoraria from Takeda, Sanofi, Janssen, Ono, Bristol-Myers Squibb. Tomoki Ito has received honoraria from Bristol-Myers Squibb and Sanofi, Grant/Research funding from Bristol-Myers Squibb. Yuji Shimura has received honoraria from Bristol-Myers Squibb, Janssen Pharmaceutical and Sanofi. Teruhito Takakuwa has received honoraria from Bristol-Myers Squib and Grant/Research funding from Janssen Pharmaceutical and Sanofi. Hirohiko Shibayama reports honoraria from Takeda, Ono, Fujimoto, Janssen, Chugai, Eisai, Sanofi, AstraZeneca, Meiji Seika Pharma, and AbbVie. Chihiro Shimazaki has received honoraria from Janssen, Bristol-Myers Squibb, and Sanofi. Junya Kuroda is a consultant for Janssen Pharmaceutical and Bristol-Myers Squibb (BMS), and has received honoraria from Janssen Pharmaceutical, Ono Pharmaceutical, Sanofi, and BMS. The other authors have no conflict of interest.
Figures

References
-
- Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394:2096–2107. doi: 10.1016/S0140-6736(19)32556-5. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

