Mitochondrial ACSS1-K635 acetylation knock-in mice exhibit altered metabolism, cell senescence, and nonalcoholic fatty liver disease
- PMID: 38758779
- PMCID: PMC11100568
- DOI: 10.1126/sciadv.adj5942
Mitochondrial ACSS1-K635 acetylation knock-in mice exhibit altered metabolism, cell senescence, and nonalcoholic fatty liver disease
Abstract
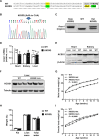
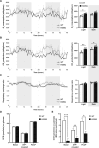
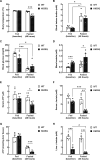
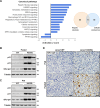
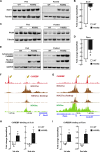
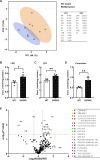
Acetyl-CoA synthetase short-chain family member 1 (ACSS1) uses acetate to generate mitochondrial acetyl-CoA and is regulated by deacetylation by sirtuin 3. We generated an ACSS1-acetylation (Ac) mimic mouse, where lysine-635 was mutated to glutamine (K635Q). Male Acss1K635Q/K635Q mice were smaller with higher metabolic rate and blood acetate and decreased liver/serum ATP and lactate levels. After a 48-hour fast, Acss1K635Q/K635Q mice presented hypothermia and liver aberrations, including enlargement, discoloration, lipid droplet accumulation, and microsteatosis, consistent with nonalcoholic fatty liver disease (NAFLD). RNA sequencing analysis suggested dysregulation of fatty acid metabolism, cellular senescence, and hepatic steatosis networks, consistent with NAFLD. Fasted Acss1K635Q/K635Q mouse livers showed increased fatty acid synthase (FASN) and stearoyl-CoA desaturase 1 (SCD1), both associated with NAFLD, and increased carbohydrate response element-binding protein binding to Fasn and Scd1 enhancer regions. Last, liver lipidomics showed elevated ceramide, lysophosphatidylethanolamine, and lysophosphatidylcholine, all associated with NAFLD. Thus, we propose that ACSS1-K635-Ac dysregulation leads to aberrant lipid metabolism, cellular senescence, and NAFLD.
Figures

References
-
- Schug Z. T., Peck B., Jones D. T., Zhang Q., Grosskurth S., Alam I. S., Goodwin L. M., Smethurst E., Mason S., Blyth K., McGarry L., James D., Shanks E., Kalna G., Saunders R. E., Jiang M., Howell M., Lassailly F., Thin M. Z., Spencer-Dene B., Stamp G., van den Broek N. J., Mackay G., Bulusu V., Kamphorst J. J., Tardito S., Strachan D., Harris A. L., Aboagye E. O., Critchlow S. E., Wakelam M. J., Schulze A., Gottlieb E., Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015). - PMC - PubMed
-
- Castro L. F., Lopes-Marques M., Wilson J. M., Rocha E., Reis-Henriques M. A., Santos M. M., Cunha I., A novel acetyl-CoA synthetase short-chain subfamily member 1 (Acss1) gene indicates a dynamic history of paralogue retention and loss in vertebrates. Gene 497, 249–255 (2012). - PubMed
-
- Morise A., Mourot J., Boue C., Combe N., Amsler G., Gripois D., Quignard-Boulange A., Yvan-Charvet L., Fenart E., Weill P., Hermier D., Gender-related response of lipid metabolism to dietary fatty acids in the hamster. Br. J. Nutr. 95, 709–720 (2006). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

