Ketogenic diet induces p53-dependent cellular senescence in multiple organs
- PMID: 38758782
- PMCID: PMC11100565
- DOI: 10.1126/sciadv.ado1463
Ketogenic diet induces p53-dependent cellular senescence in multiple organs
Abstract
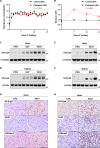
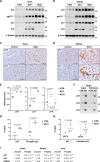
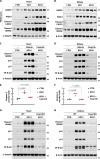
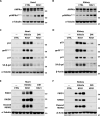
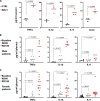
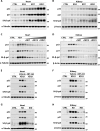
A ketogenic diet (KD) is a high-fat, low-carbohydrate diet that leads to the generation of ketones. While KDs improve certain health conditions and are popular for weight loss, detrimental effects have also been reported. Here, we show mice on two different KDs and, at different ages, induce cellular senescence in multiple organs, including the heart and kidney. This effect is mediated through adenosine monophosphate-activated protein kinase (AMPK) and inactivation of mouse double minute 2 (MDM2) by caspase-2, leading to p53 accumulation and p21 induction. This was established using p53 and caspase-2 knockout mice and inhibitors to AMPK, p21, and caspase-2. In addition, senescence-associated secretory phenotype biomarkers were elevated in serum from mice on a KD and in plasma samples from patients on a KD clinical trial. Cellular senescence was eliminated by a senolytic and prevented by an intermittent KD. These results have important clinical implications, suggesting that the effects of a KD are contextual and likely require individual optimization.
Figures


References
-
- Neal E. G., Chaffe H., Schwartz R. H., Lawson M. S., Edwards N., Fitzsimmons G., Whitney A., Cross J. H., The ketogenic diet for the treatment of childhood epilepsy: A randomised controlled trial. Lancet Neurol. 7, 500–506 (2008). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

