Catheter-based pulmonary vein isolation fails to prevent transient atrial arrhythmogenic changes related to acute obstructive respiratory events in a porcine model
- PMID: 38758963
- PMCID: PMC11167663
- DOI: 10.1093/europace/euae132
Catheter-based pulmonary vein isolation fails to prevent transient atrial arrhythmogenic changes related to acute obstructive respiratory events in a porcine model
Abstract
Aims: Pulmonary vein isolation (PVI) is the corner stone of modern rhythm control strategies in patients with atrial fibrillation (AF). Sleep-disordered breathing (SDB) is prevalent in more than 50% of patients undergoing AF ablation, and studies have indicated a greater recurrence rate after PVI in patients with SDB. Herein, we study the effect of catheter-based PVI on AF in a pig model for SDB.
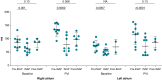
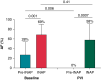
Methods and results: In 11 sedated spontaneously breathing pigs, obstructive apnoeas were simulated by 75 s of intermittent negative upper airway pressure (INAP) applied by a negative pressure device connected to the endotracheal tube. Intermittent negative upper airway pressures were performed before and after PVI. AF-inducibility and atrial effective refractory periods (aERPs) were determined before and during INAP by programmed atrial stimulation. Pulmonary vein isolation prolonged the aERP by 48 ± 27 ms in the right atrium (RA) (P < 0.0001) and by 40 ± 34 ms in the left atrium (LA) (P = 0.0004). Following PVI, AF-inducibility dropped from 28 ± 26% to 0% (P = 0.0009). Intermittent negative upper airway pressure was associated with a transient aERP-shortening (ΔaERP) in both atria, which was not prevented by PVI (INAP indued ΔaERP after PVI in the RA: -57 ± 34 ms, P = 0.0002; in the LA: -42 ± 24 ms, P < 0.0001). Intermittent negative upper airway pressure was associated with a transient increase in AF-inducibility (from 28 ± 26% to 69 ± 21%; P = 0.0008), which was not attenuated by PVI [INAP-associated AF-inducibility after PVI: 58 ± 33% (P = 0.5)].
Conclusion: Transient atrial arrhythmogenic changes related to acute obstructive respiratory events are not prevented by electrical isolation of the pulmonary veins, which partially explains the increased AF recurrence in patients with SDB after PVI procedures.
Keywords: Atrial fibrillation; Autonomic nervous system; Pulmonary vein isolation; Sleep apnoea.
© The Author(s) 2024. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures



References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. - PubMed
-
- Verhaert DVM, Betz K, Gawałko M, Hermans ANL, Pluymaekers NAHA, van der Velden RMJ et al. A VIRTUAL sleep apnoea management pathway for the work-up of atrial fibrillation patients in a digital remote infrastructure: VIRTUAL-SAFARI. Europace 2022;24:565–75. - PubMed
-
- Shukla A, Aizer A, Holmes D, Fowler S, Park DS, Bernstein S et al. Effect of obstructive sleep apnea treatment on atrial fibrillation recurrence: a meta-analysis. JACC Clin Electrophysiol 2015;1:41–51. - PubMed
-
- Kadhim K, Middeldorp ME, Elliott AD, Agbaedeng T, Gallagher C, Malik V et al. Prevalence and assessment of sleep-disordered breathing in patients with atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol 2021;37:1846–56. - PubMed
-
- Desteghe L, Hendriks JML, Heidbuchel H, Potpara TS, Lee GA, Linz D. Obstructive sleep apnoea testing and management in atrial fibrillation patients: a joint survey by the European Heart Rhythm Association (EHRA) and the Association of Cardiovascular Nurses and Allied Professions (ACNAP). Europace 2021;23:1677–84. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

