Long-Term Analysis of NRG Oncology RTOG 0415: A Randomized Phase III Noninferiority Study Comparing Two Fractionation Schedules in Patients With Low-Risk Prostate Cancer
- PMID: 38759121
- PMCID: PMC11377096
- DOI: 10.1200/JCO.23.02445
Long-Term Analysis of NRG Oncology RTOG 0415: A Randomized Phase III Noninferiority Study Comparing Two Fractionation Schedules in Patients With Low-Risk Prostate Cancer
Abstract
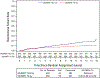
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.NRG Oncology RTOG 0415 is a randomized phase III noninferiority (NI) clinical trial comparing conventional fractionation (73.8 Gy in 41 fractions) radiotherapy (C-RT) with hypofractionation (H-RT; 70 Gy in 28) in patients with low-risk prostate cancer. The study included 1,092 protocol-eligible patients initially reported in 2016 with a median follow-up of 5.8 years. Updated results with median follow-up of 12.8 years are now presented. The estimated 12-year disease-free survival (DFS) is 56.1% (95% CI, 51.5 to 60.5) for C-RT and 61.8% (95% CI, 57.2 to 66.0) for H-RT. The DFS hazard ratio (H-RT/C-RT) is 0.85 (95% CI, 0.71 to 1.03), confirming NI (P < .001). Twelve-year cumulative incidence of biochemical failure (BF) was 17.0% (95% CI, 13.8 to 20.5) for C-RT and 9.9% (95% CI, 7.5 to 12.6) for H-RT. The HR (H-RT/C-RT) comparing biochemical recurrence between the two arms was 0.55 (95% CI, 0.39 to 0.78). Late grade ≥3 GI adverse event (AE) incidence is 3.2% (C-RT) versus 4.4% (H-RT), with relative risk (RR) for H-RT versus C-RT 1.39 (95% CI, 0.75 to 2.55). Late grade ≥3 genitourinary (GU) AE incidence is 3.4% (C-RT) versus 4.2% (H-RT), RR 1.26 (95% CI, 0.69 to 2.30). Long-term DFS is noninferior with H-RT compared with C-RT. BF is less with H-RT. No significant differences in late grade ≥3 GI/GU AEs were observed between assignments (ClinicalTrials.gov identifier: NCT00331773).
Conflict of interest statement
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at DOI
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
W. Robert Lee
James J. Dignam
This author is a member of the
Mahul B. Amin
Deborah W. Bruner
Daniel Low
David D’Souza
Jeff M. Michalski
Ian S. Dayes
William A. Hall
Paul L. Nguyen
Howard M. Sandler
No other potential conflicts of interest were reported.
Figures
References
-
- Brenner DJ, Hall EJ: Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys 43:1095–1101, 1999 - PubMed
-
- Incrocci L, Wortel RC, Alemayehu WG, et al.: Hypofractionated versus conventionally fractionated radiotherapy for patients with localised prostate cancer (HYPRO): Final efficacy results from a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol 17:1061–1069, 2016 - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous