Bioengineering methods for vascularizing organoids
- PMID: 38759654
- PMCID: PMC11228284
- DOI: 10.1016/j.crmeth.2024.100779
Bioengineering methods for vascularizing organoids
Abstract
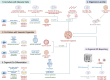
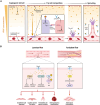
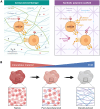
Organoids, self-organizing three-dimensional (3D) structures derived from stem cells, offer unique advantages for studying organ development, modeling diseases, and screening potential therapeutics. However, their translational potential and ability to mimic complex in vivo functions are often hindered by the lack of an integrated vascular network. To address this critical limitation, bioengineering strategies are rapidly advancing to enable efficient vascularization of organoids. These methods encompass co-culturing organoids with various vascular cell types, co-culturing lineage-specific organoids with vascular organoids, co-differentiating stem cells into organ-specific and vascular lineages, using organoid-on-a-chip technology to integrate perfusable vasculature within organoids, and using 3D bioprinting to also create perfusable organoids. This review explores the field of organoid vascularization, examining the biological principles that inform bioengineering approaches. Additionally, this review envisions how the converging disciplines of stem cell biology, biomaterials, and advanced fabrication technologies will propel the creation of increasingly sophisticated organoid models, ultimately accelerating biomedical discoveries and innovations.
Keywords: CP: Biotechnology; CP: Stem cell; bioengineering methods; human pluripotent stem cells; organoid-on-a-chip; organoids; vascularization.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests Stanford University has filed a patent application that covers the generation of vascularized organoids (US patent application numbers 63/314,958 and US2023/013997). O.J.A. is a co-founder of Bullseye Biotechnologies and a consultant for Rosebud Biosciences and CytoHub.
Figures
Similar articles
-
Developing advanced organoids: challenges, progress, and outlook.Biotechniques. 2024 Dec;76(12):575-580. doi: 10.1080/07366205.2024.2442825. Epub 2025 Jan 29. Biotechniques. 2024. PMID: 39878095
-
Getting Blood out of a Stone: Vascularization via Spheroids and Organoids in 3D Bioprinting.Cells. 2025 May 1;14(9):665. doi: 10.3390/cells14090665. Cells. 2025. PMID: 40358189 Free PMC article. Review.
-
Unlocking the full potential of human pluripotent stem cell-derived kidney organoids through bioengineering.Kidney Int. 2025 Jul;108(1):38-47. doi: 10.1016/j.kint.2025.01.043. Epub 2025 Apr 23. Kidney Int. 2025. PMID: 40280411 Review.
-
Microvascularization in 3D Human Engineered Tissue and Organoids.Annu Rev Biomed Eng. 2025 May;27(1):473-498. doi: 10.1146/annurev-bioeng-103023-115236. Annu Rev Biomed Eng. 2025. PMID: 40310885 Review.
-
Bioengineering tissue morphogenesis and function in human neural organoids.Semin Cell Dev Biol. 2021 Mar;111:52-59. doi: 10.1016/j.semcdb.2020.05.025. Epub 2020 Jun 12. Semin Cell Dev Biol. 2021. PMID: 32540123 Free PMC article. Review.
Cited by
-
Advances and Challenges of Bioassembly Strategies in Neurovascular In Vitro Modeling: An Overview of Current Technologies with a Focus on Three-Dimensional Bioprinting.Int J Mol Sci. 2024 Oct 12;25(20):11000. doi: 10.3390/ijms252011000. Int J Mol Sci. 2024. PMID: 39456783 Free PMC article. Review.
-
From gut to liver: organoids as platforms for next-generation toxicology assessment vehicles for xenobiotics.Stem Cell Res Ther. 2025 Mar 26;16(1):150. doi: 10.1186/s13287-025-04264-y. Stem Cell Res Ther. 2025. PMID: 40140938 Free PMC article. Review.
-
Stem Cells and Organoids: A Paradigm Shift in Preclinical Models Toward Personalized Medicine.Pharmaceuticals (Basel). 2025 Jul 1;18(7):992. doi: 10.3390/ph18070992. Pharmaceuticals (Basel). 2025. PMID: 40732281 Free PMC article. Review.
-
Bottom-up Biomaterial strategies for creating tailored stem cells in regenerative medicine.Front Bioeng Biotechnol. 2025 May 20;13:1581292. doi: 10.3389/fbioe.2025.1581292. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40462840 Free PMC article. Review.
-
The 3D Language of Cancer: Communication via Extracellular Vesicles from Tumor Spheroids and Organoids.Int J Mol Sci. 2025 Jul 23;26(15):7104. doi: 10.3390/ijms26157104. Int J Mol Sci. 2025. PMID: 40806235 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

