Cardiovascular outcomes in patients with homozygous familial hypercholesterolaemia on lipoprotein apheresis initiated during childhood: long-term follow-up of an international cohort from two registries
- PMID: 38759658
- PMCID: PMC11963317
- DOI: 10.1016/S2352-4642(24)00073-7
Cardiovascular outcomes in patients with homozygous familial hypercholesterolaemia on lipoprotein apheresis initiated during childhood: long-term follow-up of an international cohort from two registries
Abstract
Background: Homozygous familial hypercholesterolaemia (HoFH) is a rare genetic disease characterised by extremely high plasma LDL cholesterol from birth, causing atherosclerotic cardiovascular disease at a young age. Lipoprotein apheresis in combination with lipid-lowering drugs effectively reduce LDL cholesterol, but long-term health outcomes of such treatment are unknown. We aimed to investigate the long-term cardiovascular outcomes associated with lipoprotein apheresis initiated in childhood or adolescence.
Methods: In this cohort study, data were drawn from the HoFH International Clinical Collaboration (HICC) and the international registry for Children with Homozygous Hypercholesterolemia on Lipoprotein Apheresis (CHAIN). An overall cohort included patients diagnosed with HoFH aged 0-18 years who were alive and in follow-up between Jan 1, 2010, and Nov 8, 2021, and whose high plasma LDL cholesterol concentrations made them eligible for lipoprotein apheresis. To compare cardiovascular outcomes, patients who initiated lipoprotein apheresis in childhood (lipoprotein apheresis group) and patients who only received lipid-lowering drugs (pharmacotherapy-only group) were matched by sex and untreated plasma LDL cholesterol concentrations. The primary outcome was a composite of cardiovascular death, myocardial infarction, ischaemic stroke, percutaneous coronary intervention, coronary artery bypass grafting, aortic valve replacement, peripheral artery disease, carotid endarterectomy, angina pectoris, and supra-aortic or aortic stenosis (collectively referred to as atherosclerotic cardiovascular disease), for which survival analyses were performed in the matched cohort. Cox regression analyses were used to compare disease-free survival between cohorts and to calculate hazard ratio (HR) and 95% CI adjusted for sex, age at diagnosis, untreated plasma LDL cholesterol concentration, and number of lipid-lowering therapies other than lipoprotein apheresis.
Findings: The overall cohort included 404 patients with a median age at diagnosis of 6·0 years (IQR 3·0-9·5) and median untreated plasma LDL cholesterol of 17·8 mmol/L (14·7-20·8). The matched cohorts included 250 patients (125 patients per group), with a median untreated LDL cholesterol of 17·2 mmol/L (14·8-19·7). Mean reduction in plasma LDL cholesterol concentrations between baseline and final follow-up was greater in the lipoprotein apheresis group (-55% [95% CI -60 to -51] vs -31% [-36 to -25]; p<0·0001). Patients in the lipoprotein apheresis group had longer atherosclerotic cardiovascular disease-free survival (adjusted HR 0·52 [95% CI 0·32-0·85]) and longer cardiovascular death-free survival (0·0301 [0·0021-0·4295]). Cardiovascular death was more common in the pharmacotherapy-only group than in the lipoprotein apheresis group (ten [8%] vs one [1%]; p=0·010), whereas median age at coronary artery bypass grafting was lower in the lipoprotein apheresis group than in the pharmacotherapy-only group (15·0 years [IQR 12·0-24·0] vs 30·5 years [19·0-33·8]; p=0·037).
Interpretation: Among patients with HoFH, lipoprotein apheresis initiated during childhood and adolescence is associated with reduced long-term risk of atherosclerotic cardiovascular disease and death, and clear benefits of early initiation of high-frequency treatment on reducing plasma cholesterol were found. Consensus recommendations are now needed to guide more widespread and timely use of lipoprotein apheresis for children with HoFH, and research is required to further optimise treatment and ensure benefits of early and aggressive treatment delivery are balanced against effects on quality of life.
Funding: Amsterdam University Medical Centers, Location Academic Medical Center; Perelman School of Medicine at the University of Pennsylvania; European Atherosclerosis Society; and the US National Heart, Lung, and Blood Institute, National Institutes of Health.
Copyright © 2024 Elsevier Ltd. All rights reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Declaration of interests BAH received a research grant from Silence Therapeutics. GKH reports research grants from the Klinkerpad fonds, and part-time employment at Novo Nordisk (Søborg, Denmark) since April, 2019. GKH is shareholder of Novo Nordisk. DJB reports clinical trial fees paid to their institution from LIB Therapeutics, Novartis, Silence Therapeutics, and IONIS; speaker fees from Amgen, Sanofi, Organon, MSD, Amryt, and Novartis; and consulting fees from Amryt. ALC has received research grants or support from Amarin, Amgen, Menarini, Mylan, Sanofi, and Sanofi Regeneron, and served as a consultant for or received honoraria from Akcea, Amarin, Amgen, Daiichi Sankyo, Esperion Therapeutics, Ionis, Kowa, Medco, Menarini, MSD, Mylan, Novartis, Recordati, Regeneron, Sanofi, and The Corpus. MC reports institutional support for the conduction of clinical trials from Regeneron Pharmaceuticals and Regenxbio, and consulting fees from Amryt Pharma. AG received grants and personal fees from Amgen, Sanofi–Regeneron, Mylan Viatris, MSD, Akcea Therapeutics, Amryt, Servier, Novartis, and Ultragenyx. FJR reports receiving advisory board fees and lecture fees from Amgen, Sanofi-Aventis, Regeneron Pharmaceuticals, Novartis, and LIB Therapeutics. KKR reports grants from Amgen, Sanofi–Regeneron, Pfizer, Merck Sharp & Dohme, Daiichi Sankyo, and Ultragenyx; consulting fees from AstraZeneca, Kowa, Novartis, Sanofi, Amgen, Eli Lilly, Algorithm, Boehringer Ingelheim, Novo Nordisk, Silence Therapeutics, Bayer, Esperion, Daiichi Sankyo, Abbott, New Amsterdam, SCRIBE, CRISPR, VAXXINITY, EMENDOBIO, Cargene, Viatris, Amarin, Nodthera, and Resverlogix; honoraria for lectures from AstraZeneca, Novartis, Sanofi, Amgen, Algorithm, Boehringer Ingelheim, Novo Nordisk, Esperion, Daiichi Sankyo, and Amarin; and stock options in New Amsterdam, PEMI 31, and SCRIBE. HS reports research grants from Amgen, MSD, Synageva, Amryt, Alexion, and Akcea; consulting fees from Amgen, Alexion, Daiichi-Sankyo, Novartis, Pfizer, and Akcea; and speaker fees from Amgen, Daiichi-Sankyo, Sanofi, and Akcea. AW reports research grants from Amgen, Esperion, Novartis, Regeneron, Sanofi, Silence Therapeutics, and Ultragenyx; consulting fees from Chiesi and Novartis; and speaker fees from Algorithm, Sanofi, and Ultragenyx. All other authors declare no competing interests. The declaration of interests of individual collaborators are listed in the appendix (p 27).
Figures

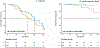
Comment in
-
Lipoprotein apheresis and long-term cardiovascular health: a real answer for children with HoFH?Lancet Child Adolesc Health. 2024 Jul;8(7):468-469. doi: 10.1016/S2352-4642(24)00105-6. Epub 2024 May 14. Lancet Child Adolesc Health. 2024. PMID: 38759659 No abstract available.
References
-
- Beheshti SO, Madsen CM, Varbo A, Nordestgaard BG. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J Am Coll Cardiol 2020;75(20):2553–66. - PubMed
-
- Cuchel M, Bruckert E, Ginsberg HN, Raal FJ, Santos RD, Hegele RA, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35(32):2146–57. - PMC - PubMed
-
- Raal FJ, Pilcher GJ, Panz VR, Deventer HEv, Brice BC, Blom DJ, Marais AD. Reduction in Mortality in Subjects With Homozygous Familial Hypercholesterolemia Associated With Advances in Lipid-Lowering Therapy. Circulation 2011;124(20):2202–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

