Alzheimer's disease pathophysiology in the Retina
- PMID: 38759947
- PMCID: PMC11285518
- DOI: 10.1016/j.preteyeres.2024.101273
Alzheimer's disease pathophysiology in the Retina
Abstract
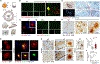
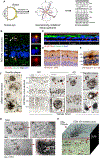
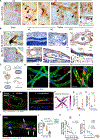
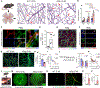
The retina is an emerging CNS target for potential noninvasive diagnosis and tracking of Alzheimer's disease (AD). Studies have identified the pathological hallmarks of AD, including amyloid β-protein (Aβ) deposits and abnormal tau protein isoforms, in the retinas of AD patients and animal models. Moreover, structural and functional vascular abnormalities such as reduced blood flow, vascular Aβ deposition, and blood-retinal barrier damage, along with inflammation and neurodegeneration, have been described in retinas of patients with mild cognitive impairment and AD dementia. Histological, biochemical, and clinical studies have demonstrated that the nature and severity of AD pathologies in the retina and brain correspond. Proteomics analysis revealed a similar pattern of dysregulated proteins and biological pathways in the retina and brain of AD patients, with enhanced inflammatory and neurodegenerative processes, impaired oxidative-phosphorylation, and mitochondrial dysfunction. Notably, investigational imaging technologies can now detect AD-specific amyloid deposits, as well as vasculopathy and neurodegeneration in the retina of living AD patients, suggesting alterations at different disease stages and links to brain pathology. Current and exploratory ophthalmic imaging modalities, such as optical coherence tomography (OCT), OCT-angiography, confocal scanning laser ophthalmoscopy, and hyperspectral imaging, may offer promise in the clinical assessment of AD. However, further research is needed to deepen our understanding of AD's impact on the retina and its progression. To advance this field, future studies require replication in larger and diverse cohorts with confirmed AD biomarkers and standardized retinal imaging techniques. This will validate potential retinal biomarkers for AD, aiding in early screening and monitoring.
Keywords: Alzheimer's disease; Inflammation; Neurodegenerative diseases; Retinal imaging; Retinal vascular pathology; Visual impairments.
Copyright © 2024. Published by Elsevier Ltd.
Conflict of interest statement
Declaration of competing interest HH declares no competing financial interests related to the present article. He is an employee of Eisai Inc. The present article has been initiated and prepared as part of his academic position at Sorbonne University, Paris, France, and reflects entirely and exclusively his own opinion. He serves as Senior Associate Editor for the Journal Alzheimer's & Dementia and does not receive any fees or honoraria since May 2019. He is inventor of 11 patents and has received no royalties: AV declares no competing financial interests related to the present article. AV contribution to this work relates to his previous academic position at Sorbonne University, Paris, France. AV was an employee of Eisai Inc. (Nov 2019–June 2021). AV does not receive any fees or honoraria since November 2019. Before November 2019 he had he received lecture honoraria from Roche, MagQu LLC, and Servier. YK and MKH are co-founding members and consultants of NeuroVision Imaging, Inc., 1395 Garden Highway, Suite 250, Sacramento, CA 95833, USA. KLB is chair and SV is CEO, co-founders and shareholders of NeuroVision Imaging, Inc., 1395 Garden Highway, Suite 250, Sacramento, CA 95833, USA. All other authors declare no competing interests related to this article.
Figures















References
-
- Abbasi J, 2017. A Retinal Scan for Alzheimer Disease. JAMA 318, ss1314. - PubMed
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T, 2000. Inflammation and Alzheimer’s disease. Neurobiol Aging 21, 383–421. - PMC - PubMed
-
- Alber J, Arthur E, Goldfarb D, Drake J, Boxerman JL, Silver B, Ott BR, Johnson LN, Snyder PJ, 2021. The relationship between cerebral and retinal microbleeds in cerebral amyloid angiopathy (CAA): A pilot study. J Neurol Sci 423, 117383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

