Fluorescent Probes for Disease Diagnosis
- PMID: 38760012
- PMCID: PMC11177268
- DOI: 10.1021/acs.chemrev.3c00776
Fluorescent Probes for Disease Diagnosis
Abstract
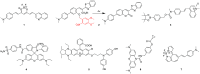
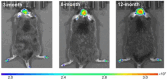
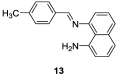
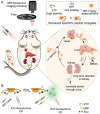
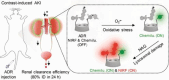
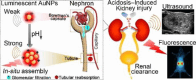
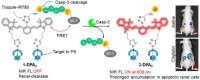
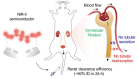
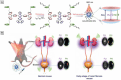
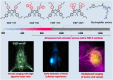
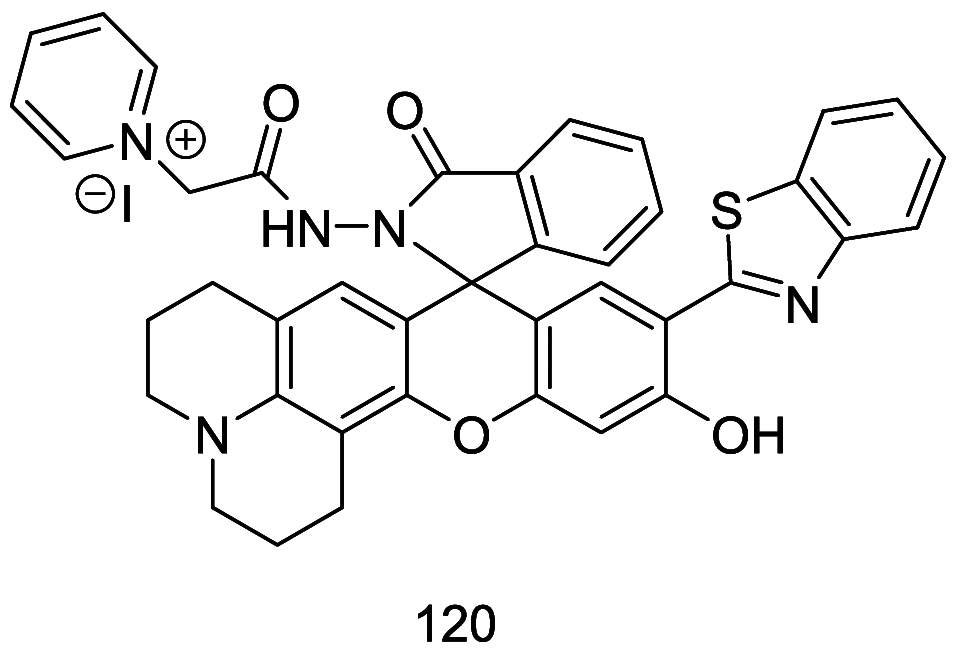
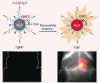
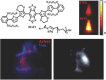
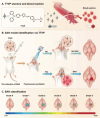
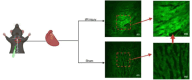
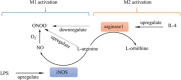
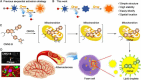
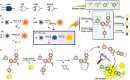
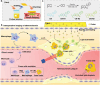
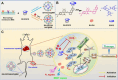
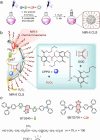
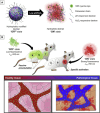
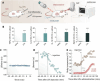
The identification and detection of disease-related biomarkers is essential for early clinical diagnosis, evaluating disease progression, and for the development of therapeutics. Possessing the advantages of high sensitivity and selectivity, fluorescent probes have become effective tools for monitoring disease-related active molecules at the cellular level and in vivo. In this review, we describe current fluorescent probes designed for the detection and quantification of key bioactive molecules associated with common diseases, such as organ damage, inflammation, cancers, cardiovascular diseases, and brain disorders. We emphasize the strategies behind the design of fluorescent probes capable of disease biomarker detection and diagnosis and cover some aspects of combined diagnostic/therapeutic strategies based on regulating disease-related molecules. This review concludes with a discussion of the challenges and outlook for fluorescent probes, highlighting future avenues of research that should enable these probes to achieve accurate detection and identification of disease-related biomarkers for biomedical research and clinical applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

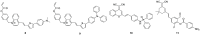



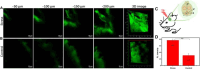
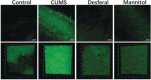
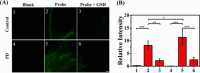












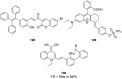
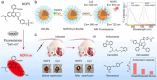
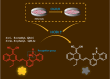
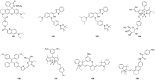






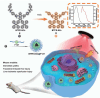
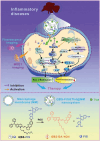

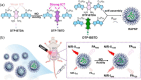
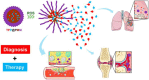




References
-
- Maire E.; Withers P. J. Quantitative X-ray Tomography. Int. Mater. Rev. 2014, 59, 1–43. 10.1179/1743280413Y.0000000023. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

