Enhancing thromboresistance of neurovascular nickel-titanium devices with responsive heparin hydrogel coatings
- PMID: 38760168
- PMCID: PMC12171471
- DOI: 10.1136/jnis-2024-021836
Enhancing thromboresistance of neurovascular nickel-titanium devices with responsive heparin hydrogel coatings
Abstract
Background: Neurointerventional devices, particularly laser-cut thin-strut stents made of self-expanding nickel-titanium alloy, are increasingly utilized for endovascular applications in intracranial arteries and dural venous sinuses. Preventing thrombosis and stroke necessitates systemic anticoagulant and antiplatelet therapies with the risk of bleeding complications. Antithrombotic coatings present a promising solution.
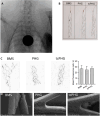
Methods: In this study, we investigated the potential of hydrogels composed of four-armed poly(ethylene glycol) (starPEG) and heparin, with or without coagulation-responsive heparin release, as coatings for neurovascular devices to mitigate blood clot formation. We evaluated the feasibility and efficacy of these coatings on neurovascular devices through in vitro Chandler-Loop assays and implantation experiments in the supra-aortic arteries of rabbits.
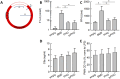
Results: Stable and coagulation-responsive starPEG-heparin hydrogel coatings exhibited antithrombotic efficacy in vitro, although with a slightly reduced thromboprotection observed in vivo. Furthermore, the hydrogel coatings demonstrated robustness against shear forces encountered during deployment and elicited only marginal humoral and cellular inflammatory responses compared with the reference standards.
Conclusion: Heparin hydrogel coatings offer promising benefits for enhancing the hemocompatibility of neurointerventional devices made of self-expanding nickel-titanium alloy. The variance in performance between in vitro and in vivo settings may be attributed to differences in low- and high-shear blood flow conditions inherent to these models. These models may represent the differences in venous and arterial systems. Further optimization is warranted to tailor the hydrogel coatings for improved efficacy in arterial applications.
Keywords: Material; Pharmacology; Stent.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: Matthias Gawlitza reports consultancy agreements with MicroVention, Balt, Phenox. Scientific advisory board of Simq. The other authors declare that they have no competing interests.
Figures


Similar articles
-
Poly(ethylene glycol)-norbornene-heparin-piposome composite hydrogels for in situ spraying and ultra-fast adhesion: meeting the challenges of endothelial repair of vascular injury.Acta Biomater. 2025 Jun 15;200:489-507. doi: 10.1016/j.actbio.2025.04.060. Epub 2025 May 15. Acta Biomater. 2025. PMID: 40381928
-
Analysis of frictional forces in experimental models of stent retriever mechanical thrombectomy.J Biomech. 2024 Feb;164:111971. doi: 10.1016/j.jbiomech.2024.111971. Epub 2024 Feb 1. J Biomech. 2024. PMID: 38309134
-
Antiadhesive and Antibacterial Coatings for Short-Term Titanium Implants.Macromol Rapid Commun. 2025 Jun;46(12):e2400989. doi: 10.1002/marc.202400989. Epub 2025 Mar 8. Macromol Rapid Commun. 2025. PMID: 40056084 Free PMC article.
-
Development of new anticoagulants targeting coagulation factor XI and prospects for clinical use.J Cardiol. 2025 Jun;85(6):473-479. doi: 10.1016/j.jjcc.2025.02.013. Epub 2025 Feb 14. J Cardiol. 2025. PMID: 39954726 Review.
-
Comparison of the Safety and Efficacy of Direct Oral Anticoagulants and Warfarin in Atrial Fibrillation or Venous Thromboembolism in Patients with Renal Impairment: Systematic Review, Meta-Analysis and Network Meta-Analysis.Am J Cardiovasc Drugs. 2021 Nov;21(6):643-657. doi: 10.1007/s40256-021-00469-7. Epub 2021 Apr 4. Am J Cardiovasc Drugs. 2021. PMID: 33817758
Cited by
-
Smart materials strategy for vascular challenges targeting in-stent restenosis: a critical review.Regen Biomater. 2025 Mar 24;12:rbaf020. doi: 10.1093/rb/rbaf020. eCollection 2025. Regen Biomater. 2025. PMID: 40290450 Free PMC article. Review.
References
-
- Pierot L, Barbe C, Nguyen HA, et al. Intraoperative complications of Endovascular treatment of intracranial aneurysms with coiling or balloon-assisted coiling in a prospective multicenter cohort of 1088 participants: analysis of Recanalization after Endovascular treatment of intracranial aneurysm (ARETA) study. Radiology. 2020;295:381–9. doi: 10.1148/radiol.2020191842. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
