Liver ACOX1 regulates levels of circulating lipids that promote metabolic health through adipose remodeling
- PMID: 38760332
- PMCID: PMC11101658
- DOI: 10.1038/s41467-024-48471-2
Liver ACOX1 regulates levels of circulating lipids that promote metabolic health through adipose remodeling
Abstract
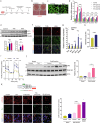
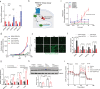
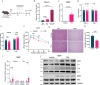
The liver gene expression of the peroxisomal β-oxidation enzyme acyl-coenzyme A oxidase 1 (ACOX1), which catabolizes very long chain fatty acids (VLCFA), increases in the context of obesity, but how this pathway impacts systemic energy metabolism remains unknown. Here, we show that hepatic ACOX1-mediated β-oxidation regulates inter-organ communication involved in metabolic homeostasis. Liver-specific knockout of Acox1 (Acox1-LKO) protects mice from diet-induced obesity, adipose tissue inflammation, and systemic insulin resistance. Serum from Acox1-LKO mice promotes browning in cultured white adipocytes. Global serum lipidomics show increased circulating levels of several species of ω-3 VLCFAs (C24-C28) with previously uncharacterized physiological role that promote browning, mitochondrial biogenesis and Glut4 translocation through activation of the lipid sensor GPR120 in adipocytes. This work identifies hepatic peroxisomal β-oxidation as an important regulator of metabolic homeostasis and suggests that manipulation of ACOX1 or its substrates may treat obesity-associated metabolic disorders.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
- R01 DK133344/DK/NIDDK NIH HHS/United States
- P30 DK056341/DK/NIDDK NIH HHS/United States
- DK132239/U.S. Department of Health & Human Services | NIH | Office of Extramural Research, National Institutes of Health (OER)
- T32 DK007120/DK/NIDDK NIH HHS/United States
- R01 DK132239/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

