Active droplets through enzyme-free, dynamic phosphorylation
- PMID: 38760374
- PMCID: PMC11101487
- DOI: 10.1038/s41467-024-48571-z
Active droplets through enzyme-free, dynamic phosphorylation
Abstract
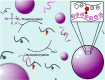
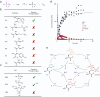
Life continuously transduces energy to perform critical functions using energy stored in reactive molecules like ATP or NADH. ATP dynamically phosphorylates active sites on proteins and thereby regulates their function. Inspired by such machinery, regulating supramolecular functions using energy stored in reactive molecules has gained traction. Enzyme-free, synthetic systems that use dynamic phosphorylation to regulate supramolecular processes have not yet been reported, to our knowledge. Here, we show an enzyme-free reaction cycle that consumes the phosphorylating agent monoamidophosphate by transiently phosphorylating histidine and histidine-containing peptides. The phosphorylated species are labile and deactivate through hydrolysis. The cycle exhibits versatility and tunability, allowing for the dynamic phosphorylation of multiple precursors with a tunable half-life. Notably, we show the resulting phosphorylated products can regulate the peptide's phase separation, leading to active droplets that require the continuous conversion of fuel to sustain. The reaction cycle will be valuable as a model for biological phosphorylation but can also offer insights into protocell formation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
MeSH terms
LinkOut - more resources
Full Text Sources

