Sustained-release drug delivery systems
- PMID: 38760462
- PMCID: PMC11885436
- DOI: 10.1038/s41433-024-03134-w
Sustained-release drug delivery systems
Abstract
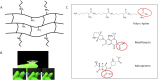
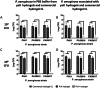
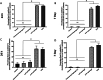
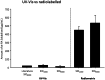
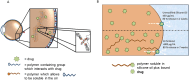
The design and development of a sustained-release drug delivery system targeting the administration of active pharmaceutical ingredients (APIs) to the eye could overcome the limitations of topically administered eye drops. Understanding how to modify or design new materials with specific functional properties that promote the attachment and release of specific drugs over longer time periods, alongside understanding clinical needs, can lead to new strategic opportunities to improve treatment options. In this paper we discuss two approaches to the design or modification of materials to produce a sustained therapeutic effect. Firstly, we discuss how the synthesis of a peptide hydrogel from a naturally-derived antimicrobial material led to the design of a bandage contact lens which may be able to be used prophylactically to reduce post-surgery infection. Secondly, we discuss how silicone oil tamponade agents used to treat retinal detachments can have adjunctive behaviour to enhance the solubility of the anti-proliferative drug retinoic acid and produce a sustained release over several weeks. These studies are the result of close partnerships between clinical ophthalmologists, materials scientists, and chemists, and illustrate how these partnerships can lead to comprehensive understandings that have the potential to change patient outcomes.
摘要: 设计和开发针对眼部有效药物成分(APIs)给药的缓释药物递送系统, 可克服局部滴眼液的局限性。了解如何修饰或设计具有特定功能特性的新材料, 以促进特定药物在更长时间段内的附着和释放, 并且满足临床需求, 可带来新的改进治疗方案的战略机遇。本文讨论了两种设计或改进的材料以产生持续治疗效果的方法。首先, 我们讨论了如何用天然抗菌材料合成的多肽水凝胶设计绷带镜, 这种绷带镜可预防性地用于减少术后感染。其次, 我们讨论了用于治疗视网膜脱离的硅油填充剂如何具有辅助作用, 以增强抗增殖药物维甲酸的溶解性, 并在数周内持续释放。这些成果得益于临床眼科医生、材料学家和化学家之间的密切合作, 文中阐明了如何加深理解这些合作关系以改变患者的临床预后。.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors are co-inventors of patents (EP3496759A1, US20190175742A1; US20190175497A1) that describe some of the work reported here.
Figures



Similar articles
-
Tamponade in surgery for retinal detachment associated with proliferative vitreoretinopathy.Cochrane Database Syst Rev. 2020 May 13;5(5):CD006126. doi: 10.1002/14651858.CD006126.pub4. Cochrane Database Syst Rev. 2020. PMID: 32408387 Free PMC article.
-
Challenges and opportunities for drug delivery to the posterior of the eye.Drug Discov Today. 2019 Aug;24(8):1679-1684. doi: 10.1016/j.drudis.2019.05.035. Epub 2019 Jun 5. Drug Discov Today. 2019. PMID: 31175955 Free PMC article. Review.
-
An update on the latest strategies in retinal drug delivery.Expert Opin Drug Deliv. 2024 May;21(5):695-712. doi: 10.1080/17425247.2024.2358886. Epub 2024 May 27. Expert Opin Drug Deliv. 2024. PMID: 38787783 Review.
-
Controlled and continuous release ocular drug delivery systems: pros and cons.Curr Drug Deliv. 2012 Jul;9(4):421-30. doi: 10.2174/156720112801323125. Curr Drug Deliv. 2012. PMID: 22640036 Review.
-
Drug delivery to the posterior segment of the eye.Eur J Ophthalmol. 2011;21 Suppl 6:S20-6. doi: 10.5301/EJO.2010.6051. Eur J Ophthalmol. 2011. PMID: 23264325 Review.
Cited by
-
Colloid-Forming Prodrug-Hydrogel Composite Prolongs Lower Intraocular Pressure in Rodent Eyes after Subconjunctival Injection.Adv Mater. 2025 Feb;37(8):e2419306. doi: 10.1002/adma.202419306. Epub 2025 Jan 6. Adv Mater. 2025. PMID: 39763100 Free PMC article.
-
Minimizing Oxidative Stress in the Lens: Alternative Measures for Elevating Glutathione in the Lens to Protect against Cataract.Antioxidants (Basel). 2024 Oct 1;13(10):1193. doi: 10.3390/antiox13101193. Antioxidants (Basel). 2024. PMID: 39456447 Free PMC article. Review.
-
Trends in Photopolymerization 3D Printing for Advanced Drug Delivery Applications.Biomacromolecules. 2025 Jan 13;26(1):85-117. doi: 10.1021/acs.biomac.4c01004. Epub 2024 Dec 3. Biomacromolecules. 2025. PMID: 39625843 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

