Dual-energy lattice-tip ablation system for persistent atrial fibrillation: a randomized trial
- PMID: 38760584
- PMCID: PMC11333282
- DOI: 10.1038/s41591-024-03022-6
Dual-energy lattice-tip ablation system for persistent atrial fibrillation: a randomized trial
Abstract
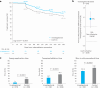
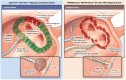
Clinical outcomes of catheter ablation for atrial fibrillation (AF) are suboptimal due, in part, to challenges in achieving durable lesions. Although focal point-by-point ablation allows for the creation of any required lesion set, this strategy necessitates the generation of contiguous lesions without gaps. A large-tip catheter, capable of creating wide-footprint ablation lesions, may increase ablation effectiveness and efficiency. In a randomized, single-blind, non-inferiority trial, 420 patients with persistent AF underwent ablation using a large-tip catheter with dual pulsed field and radiofrequency energies versus ablation using a conventional radiofrequency ablation system. The primary composite effectiveness endpoint was evaluated through 1 year and included freedom from acute procedural failure and repeat ablation at any time, plus arrhythmia recurrence, drug initiation or escalation or cardioversion after a 3-month blanking period. The primary safety endpoint was freedom from a composite of serious procedure-related or device-related adverse events. The primary effectiveness endpoint was observed for 73.8% and 65.8% of patients in the investigational and control arms, respectively (P < 0.0001 for non-inferiority). Major procedural or device-related complications occurred in three patients in the investigational arm and in two patients in the control arm (P < 0.0001 for non-inferiority). In a secondary analysis, procedural times were shorter in the investigational arm as compared to the control arm (P < 0.0001). These results demonstrate non-inferior safety and effectiveness of the dual-energy catheter for the treatment of persistent AF. Future large-scale studies are needed to gather real-world evidence on the impact of the focal dual-energy lattice catheter on the broader population of patients with AF. ClinicalTrials.gov identifier: NCT05120193 .
© 2024. The Author(s).
Conflict of interest statement
E.A. is a consultant to and has received equity from Affera-Medtronic. Unrelated to this manuscript, he serves in consulting and advisory capacities for Biosense Webster, Boston Scientific and Abbott Medical. He has received research grants from Biosense Webster and Medtronic. V.Y.R. is a consultant to and has received equity from Affera-Medtronic. Unrelated to this manuscript, V.Y.R. has served as a consultant for and has equity in Ablacon, Acutus Medical, Anumana, Apama Medical-Boston Scientific, APN Health, Aquaheart, Atacor, Autonomix, Axon Therapies, Backbeat, BioSig, CardiaCare, Cardiofocus, CardioNXT/AFTx, Circa Scientific, CoRISMA, Corvia Medical, Dinova-Hangzhou DiNovA EP Technology, East End Medical, EPD-Philips, EP Frontiers, Epix Therapeutics-Medtronic, EpiEP, Eximo, Farapulse-Boston Scientific, Field Medical, Focused Therapeutics, HRT, Intershunt, Javelin, Kardium, Keystone Heart, Laminar Medical, LuxMed, Medlumics, Middlepeak, Neutrace, Nuvera-Biosense Webster, Oracle Health, Restore Medical, Sirona Medical, SoundCath and Valcare. Unrelated to this work, V.Y.R. has served as a consultant for Abbott, Adagio Medical, Append Medical, AtriAN, Biosense Webster, BioTel Heart, Biotronik, Boston Scientific, Cairdac, Cardionomic, CoreMap, Fire1, Gore & Associates, Impulse Dynamics, Medtronic, Novartis, Novo Nordisk, Philips and Pulse Biosciences. Unrelated to this work, V.Y.R. has equity in Atraverse, DRS Vascular, Manual Surgical Sciences, Newpace, Nyra Medical, Surecor and Vizaramed. A.N. has served as a consultant for iRhythm, Boston Scientific, Biosense Webster, Abbott and Biotronik. M.M. has served as a consultant for Boston Scientific, Biosense Webster, Abbott, Medtronic, Siemens and Sentre Heart/Atricure and has equity in EPD-Philips (divested) and NewPace, Ltd. A.A. has served in consulting and advisory capacities for Medtronic, Boston Scientific and Biosense Webster and in medical education for Siemens. A.A. has equity in Biostar Ventures and has served in consulting and medical education for and been supported by research grants from Philips. S.M. has received Medtronic research grants and honoraria. T.T. has served as a consultant for Biosense Webster and Medtronic. D.N. reports the following disclosures: Abbott Medical: consultant, advisory board and research grants; Boston Scientific: consultant, advisory board and research grants; Medtronic: consultant, advisory board and research grants; Biosense Webster: consultant, advisory board and research grants; Adagio: consultant and research grants; Laminar: research grants; and TerraRecon: consultant. E.K. has served in consulting and advisory capacities for Biosense Webster, Medtronic and Philips, unrelated to this manuscript or technology. J.K. reports personal fees from Biosense Webster, Boston Scientific, GE Healthcare, Medtronic and St. Jude Medical (Abbott) for participation in scientific advisory boards and has received speaker honoraria from Biosense Webster, Biotronik, Boston Scientific, Medtronic and St. Jude Medical (Abbott). D.S. reports grant/research support from Medtronic and consultant/advisory board participation for Biosense Webster, Medtronic, EBR Inc., AltaThera Pharmaceutical and Attune Medical. J.O. reports the following disclosures: Medtronic: consulting; Biosense Webster: research grants, consulting and advisory board; Boston Scientific: consulting, research grants and advisory board; and Abbott: consulting and research grants. J.H. has served as a consultant for Medtronic, Abbott and Volta. D.H., P.H., S.L., B.O. and K.G.T. are employees of Medtronic. The remaining authors declare no competing interests.
Figures
References
-
- Hindricks, G. et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J.42, 373–498 (2021). - PubMed
-
- Haissaguerre, M. et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N. Engl. J. Med.339, 659–666 (1998). - PubMed
-
- Mansour, M. et al. Persistent atrial fibrillation ablation with contact force-sensing catheter: the prospective multicenter PRECEPT trial. JACC Clin. Electrophysiol.6, 958–969 (2020). - PubMed
-
- Verma, A. et al. Approaches to catheter ablation for persistent atrial fibrillation. N. Engl. J. Med.372, 1812–1822 (2015). - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

