A prognostic neural epigenetic signature in high-grade glioma
- PMID: 38760585
- PMCID: PMC11186787
- DOI: 10.1038/s41591-024-02969-w
A prognostic neural epigenetic signature in high-grade glioma
Abstract
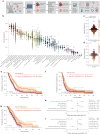
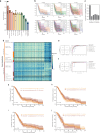
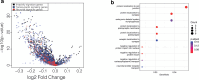
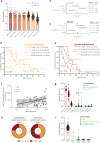
Neural-tumor interactions drive glioma growth as evidenced in preclinical models, but clinical validation is limited. We present an epigenetically defined neural signature of glioblastoma that independently predicts patients' survival. We use reference signatures of neural cells to deconvolve tumor DNA and classify samples into low- or high-neural tumors. High-neural glioblastomas exhibit hypomethylated CpG sites and upregulation of genes associated with synaptic integration. Single-cell transcriptomic analysis reveals a high abundance of malignant stemcell-like cells in high-neural glioblastoma, primarily of the neural lineage. These cells are further classified as neural-progenitor-cell-like, astrocyte-like and oligodendrocyte-progenitor-like, alongside oligodendrocytes and excitatory neurons. In line with these findings, high-neural glioblastoma cells engender neuron-to-glioma synapse formation in vitro and in vivo and show an unfavorable survival after xenografting. In patients, a high-neural signature is associated with decreased overall and progression-free survival. High-neural tumors also exhibit increased functional connectivity in magnetencephalography and resting-state magnet resonance imaging and can be detected via DNA analytes and brain-derived neurotrophic factor in patients' plasma. The prognostic importance of the neural signature was further validated in patients diagnosed with diffuse midline glioma. Our study presents an epigenetically defined malignant neural signature in high-grade gliomas that is prognostically relevant. High-neural gliomas likely require a maximized surgical resection approach for improved outcomes.
© 2024. The Author(s).
Conflict of interest statement
M.L.S. is an equity holder, scientific co-founder and advisory board member of Immunitas Therapeutics. M.M. holds equity in MapLight Therapeutics. The other authors declare no competing interests.
Figures











References
MeSH terms
Grants and funding
- R01NS092597/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- 310030 185155/1/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- DP1 NS111132/NS/NINDS NIH HHS/United States
- R01 NS092597/NS/NINDS NIH HHS/United States
- R21 CA264339/CA/NCI NIH HHS/United States
- HE 8145/6-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 74259/Robert Wood Johnson Foundation (RWJF)
- SFB 824 B12/Deutsche Forschungsgemeinschaft (German Research Foundation)
- K08NS110919/U.S. Department of Health & Human Services | NIH | NCI | Division of Cancer Epidemiology and Genetics, National Cancer Institute (National Cancer Institute Division of Cancer Epidemiology and Genetics)
- R01CA258384/U.S. Department of Health & Human Services | NIH | NCI | Division of Cancer Epidemiology and Genetics, National Cancer Institute (National Cancer Institute Division of Cancer Epidemiology and Genetics)
- K08 NS110919/NS/NINDS NIH HHS/United States
- HE 8145/5-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- U19 CA264504/CA/NCI NIH HHS/United States
- R01 CA258384/CA/NCI NIH HHS/United States
- U19CA264504/U.S. Department of Health & Human Services | NIH | NCI | Division of Cancer Epidemiology and Genetics, National Cancer Institute (National Cancer Institute Division of Cancer Epidemiology and Genetics)
- SFB 1389/Deutsche Forschungsgemeinschaft (German Research Foundation)
- P50 CA097257/CA/NCI NIH HHS/United States
- SFB 1192 B8/Deutsche Forschungsgemeinschaft (German Research Foundation)
- DFG RI2616/3-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- R01 NS137950/NS/NINDS NIH HHS/United States
- SFB 1192 C3/Deutsche Forschungsgemeinschaft (German Research Foundation)
- P50 CA165962/CA/NCI NIH HHS/United States
- P50CA097257/U.S. Department of Health & Human Services | NIH | NCI | Division of Cancer Epidemiology and Genetics, National Cancer Institute (National Cancer Institute Division of Cancer Epidemiology and Genetics)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

