p75 neurotrophin receptor modulation in mild to moderate Alzheimer disease: a randomized, placebo-controlled phase 2a trial
- PMID: 38760589
- PMCID: PMC11186782
- DOI: 10.1038/s41591-024-02977-w
p75 neurotrophin receptor modulation in mild to moderate Alzheimer disease: a randomized, placebo-controlled phase 2a trial
Abstract
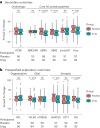
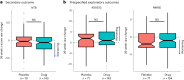
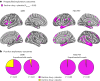
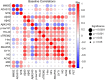
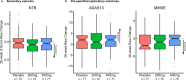
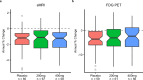
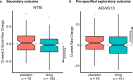
p75 neurotrophin receptor (p75NTR) signaling pathways substantially overlap with degenerative networks active in Alzheimer disease (AD). Modulation of p75NTR with the first-in-class small molecule LM11A-31 mitigates amyloid-induced and pathological tau-induced synaptic loss in preclinical models. Here we conducted a 26-week randomized, placebo-controlled, double-blinded phase 2a safety and exploratory endpoint trial of LM11A-31 in 242 participants with mild to moderate AD with three arms: placebo, 200 mg LM11A-31 and 400 mg LM11A-31, administered twice daily by oral capsules. This trial met its primary endpoint of safety and tolerability. Within the prespecified secondary and exploratory outcome domains (structural magnetic resonance imaging, fluorodeoxyglucose positron-emission tomography and cerebrospinal fluid biomarkers), significant drug-placebo differences were found, consistent with the hypothesis that LM11A-31 slows progression of pathophysiological features of AD; no significant effect of active treatment was observed on cognitive tests. Together, these results suggest that targeting p75NTR with LM11A-31 warrants further investigation in larger-scale clinical trials of longer duration. EU Clinical Trials registration: 2015-005263-16 ; ClinicalTrials.gov registration: NCT03069014 .
© 2024. The Author(s).
Conflict of interest statement
K.C. is a consultant to ADM Diagnostics. E.M.R. is a compensated scientific advisor to Alzheon, Aural Analytics, Cognition Therapeutics, Denali, Enigma, Retromer Therapeutics and Vaxxinity and a cofounder and advisor of ALZPath. K.B. has provided consultation to Abbvie, AC Immune, AriBio, ALZpath, BioArctic, Biogen, Eisai, Lilly, Ono Pharma, Prothena, Roche Diagnostics and Siemens Healthineers. K.B. has participated in a data safety monitoring board or advisory board for Julius Clinical and Novartis. K.B. has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics. K.B. is a cofounder of and has stock in Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. J.L.C. has provided consultation to Acadia, Actinogen, Acumen, AlphaCognition, Aprinoia, Artery, Biogen, Biohaven, BioXcel, Bristol-Myers Squib, Cassava, Cerecin, Eisai, GAP Foundation, Janssen, Karuna, Lighthouse, Lilly, Lundbeck, EQT (formerly LSP), Merck, NervGen, New Amsterdam, Novo Nordisk, Oligomerix, Optoceutics, Ono, Otsuka, Oxford Brain Diagnostics, PharmatrophiX, Prothena, ReMYND, Roche, Sage Therapeutics, Scottish Brain Science, Signant Health, Simcere, sinaptica, Suven, TrueBinding, Vaxxinity and Wren pharmaceutical, assessment and investment companies. F.M.L. and S.M.M. are listed as inventors on patents related to LM11A-31 that are assigned to the University of North Carolina, University of California, San Francisco and the Department of Veterans Affairs. They are also entitled to royalties distributed by UC and the VA per their standard agreements. F.M.L. is a principal of and has financial interest in PharmatrophiX, a company focused on the development of small-molecule ligands for neurotrophin receptors, with licensing of several related patents. M.W. is the vice president for research and development of NeuroScios. The other authors declare no competing interests.
Figures




References
-
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- R35 AG071476/AG/NIA NIH HHS/United States
- P30 AG072980/AG/NIA NIH HHS/United States
- SG-23-1038904 QC/ALZ/Alzheimer's Association/United States
- 2022-00732/Vetenskapsrådet (Swedish Research Council)
- P20 GM109025/GM/NIGMS NIH HHS/United States
- R01 AG053798/AG/NIA NIH HHS/United States
- R35AG71476/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- ZEN-21-848495/ALZ/Alzheimer's Association/United States
- R01 AG051596/AG/NIA NIH HHS/United States
- P20GM109025/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- 453677/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- P20 AG068053/AG/NIA NIH HHS/United States
- 2017-00915/Vetenskapsrådet (Swedish Research Council)
- U01 AG024904/AG/NIA NIH HHS/United States
- R01AG053798/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R25 AG083721-01/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R25 AG083721/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

