Functional extracellular vesicles from SHEDs combined with gelatin methacryloyl promote the odontogenic differentiation of DPSCs for pulp regeneration
- PMID: 38760763
- PMCID: PMC11102175
- DOI: 10.1186/s12951-024-02542-0
Functional extracellular vesicles from SHEDs combined with gelatin methacryloyl promote the odontogenic differentiation of DPSCs for pulp regeneration
Abstract
Background: Pulp regeneration is a novel approach for the treatment of immature permanent teeth with pulp necrosis. This technique includes the combination of stem cells, scaffolds, and growth factors. Recently, stem cell-derived extracellular vesicles (EVs) have emerged as a new methodology for pulp regeneration. Emerging evidence has proven that preconditioning is an effective scheme to modify EVs for better therapeutic potency. Meanwhile, proper scaffolding is of great significance to protect EVs from rapid clearance and destruction. This investigation aims to fabricate an injectable hydrogel loaded with EVs from pre-differentiated stem cells from human exfoliated deciduous teeth (SHEDs) and examine their effects on pulp regeneration.
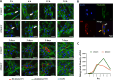
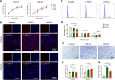
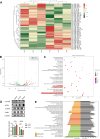
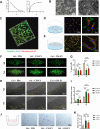
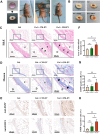
Results: We successfully employed the odontogenic induction medium (OM) of SHEDs to generate functional EV (OM-EV). The OM-EV at a concentration of 20 µg/mL was demonstrated to promote the proliferation and migration of dental pulp stem cells (DPSCs). The results revealed that OM-EV has a better potential to promote odontogenic differentiation of DPSCs than common EVs (CM-EV) in vitro through Alizarin red phalloidin, alkaline phosphatase staining, and assessment of the expression of odontogenic-related markers. High-throughput sequencing suggests that the superior effects of OM-EV may be attributed to activation of the AMPK/mTOR pathway. Simultaneously, we prepared a photocrosslinkable gelatin methacryloyl (GelMA) to construct an OM-EV-encapsulated hydrogel. The hydrogel exhibited sustained release of OM-EV and good biocompatibility for DPSCs. The released OM-EV from the hydrogel could be internalized by DPSCs, thereby enhancing their survival and migration. In tooth root slices that were subcutaneously transplanted in nude mice, the OM-EV-encapsulated hydrogel was found to facilitate dentinogenesis. After 8 weeks, there was more formation of mineralized tissue, as well as higher levels of dentin sialophosphoprotein (DSPP) and dentin matrix protein-1 (DMP-1).
Conclusions: The effects of EV can be substantially enhanced by preconditioning of SHEDs. The functional EVs from SHEDs combined with GelMA are capable of effectively promoting dentinogenesis through upregulating the odontogenic differentiation of DPSCs, which provides a promising therapeutic approach for pulp regeneration.
Keywords: Extracellular vesicles; Hydrogel; Odontogenic differentiation; Pulp regeneration; Stem cells from human exfoliated deciduous teeth.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Xuan K, Li B, Guo H, Sun W, Kou X, He X, et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci Transl Med. 2018;10:eaaf3227. - PubMed
-
- Wu J, Chen L, Wang R, Song Z, Shen Z, Zhao Y, Huang S, Lin Z. Exosomes secreted by stem cells from human exfoliated deciduous teeth promote alveolar bone defect repair through the regulation of angiogenesis and osteogenesis. Acs Biomater Sci Eng. 2019;5:3561–71. doi: 10.1021/acsbiomaterials.9b00607. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

