Neutrophils in Cancer immunotherapy: friends or foes?
- PMID: 38760815
- PMCID: PMC11102125
- DOI: 10.1186/s12943-024-02004-z
Neutrophils in Cancer immunotherapy: friends or foes?
Abstract
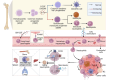
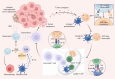
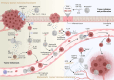
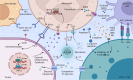
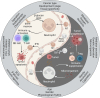
Neutrophils play a Janus-faced role in the complex landscape of cancer pathogenesis and immunotherapy. As immune defense cells, neutrophils release toxic substances, including reactive oxygen species and matrix metalloproteinase 9, within the tumor microenvironment. They also modulate the expression of tumor necrosis factor-related apoptosis-inducing ligand and Fas ligand, augmenting their capacity to induce tumor cell apoptosis. Their involvement in antitumor immune regulation synergistically activates a network of immune cells, bolstering anticancer effects. Paradoxically, neutrophils can succumb to the influence of tumors, triggering signaling cascades such as JAK/STAT, which deactivate the immune system network, thereby promoting immune evasion by malignant cells. Additionally, neutrophil granular constituents, such as neutrophil elastase and vascular endothelial growth factor, intricately fuel tumor cell proliferation, metastasis, and angiogenesis. Understanding the mechanisms that guide neutrophils to collaborate with other immune cells for comprehensive tumor eradication is crucial to enhancing the efficacy of cancer therapeutics. In this review, we illuminate the underlying mechanisms governing neutrophil-mediated support or inhibition of tumor progression, with a particular focus on elucidating the internal and external factors that influence neutrophil polarization. We provide an overview of recent advances in clinical research regarding the involvement of neutrophils in cancer therapy. Moreover, the future prospects and limitations of neutrophil research are discussed, aiming to provide fresh insights for the development of innovative cancer treatment strategies targeting neutrophils.
Keywords: Antitumor activity; Cancer; Immunotherapy; Neutrophils; Protumor activity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

