CCT6A alleviates pulmonary fibrosis by inhibiting HIF-1α-mediated lactate production
- PMID: 38760881
- PMCID: PMC11574388
- DOI: 10.1093/jmcb/mjae021
CCT6A alleviates pulmonary fibrosis by inhibiting HIF-1α-mediated lactate production
Abstract
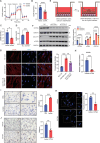
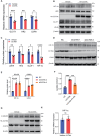
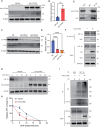
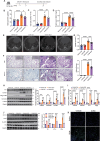
Idiopathic pulmonary fibrosis (IPF) is a lethal progressive fibrotic lung disease. The development of IPF involves different molecular and cellular processes, and recent studies indicate that lactate plays a significant role in promoting the progression of the disease. Nevertheless, the mechanism by which lactate metabolism is regulated and the downstream effects remain unclear. The molecular chaperone CCT6A performs multiple functions in a variety of biological processes. Our research has identified a potential association between CCT6A and serum lactate levels in IPF patients. Herein, we found that CCT6A was highly expressed in type 2 alveolar epithelial cells (AEC2s) of fibrotic lung tissues and correlated with disease severity. Lactate increases the accumulation of lipid droplets in epithelial cells. CCT6A inhibits lipid synthesis by blocking the production of lactate in AEC2s and alleviates bleomycin-induced pulmonary fibrosis in mice. In addition, our results revealed that CCT6A blocks HIF-1α-mediated lactate production by driving the VHL-dependent ubiquitination and degradation of HIF-1α and further inhibits lipid accumulation in fibrotic lungs. In conclusion, we propose that there is a pivotal regulatory role of CCT6A in lactate metabolism in pulmonary fibrosis, and strategies aimed at targeting these key molecules could represent potential therapeutic approaches for pulmonary fibrosis.
Keywords: CCT6A; HIF-1α; IPF; lactate signaling; metabolism.
© The Author(s) (2024). Published by Oxford University Press on behalf of Journal of Molecular Cell Biology, CEMCS, CAS.
Figures



References
-
- Ahmed K., Tunaru S., Tang C. et al. (2010). An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell Metab. 11, 311–319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

