Hydroxycitrate delays early mortality in mice and promotes muscle regeneration while inducing a rich hepatic energetic status
- PMID: 38760909
- PMCID: PMC11488303
- DOI: 10.1111/acel.14205
Hydroxycitrate delays early mortality in mice and promotes muscle regeneration while inducing a rich hepatic energetic status
Abstract
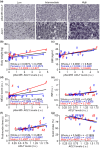
ATP citrate lyase (ACLY) inhibitors have the potential of modulating central processes in protein, carbohydrate, and lipid metabolism, which can have relevant physiological consequences in aging and age-related diseases. Here, we show that hepatic phospho-active ACLY correlates with overweight and Model for End-stage Liver Disease score in humans. Wild-type mice treated chronically with the ACLY inhibitor potassium hydroxycitrate exhibited delayed early mortality. In AML12 hepatocyte cultures, the ACLY inhibitors potassium hydroxycitrate, SB-204990, and bempedoic acid fostered lipid accumulation, which was also observed in the liver of healthy-fed mice treated with potassium hydroxycitrate. Analysis of soleus tissue indicated that potassium hydroxycitrate produced the modulation of wound healing processes. In vivo, potassium hydroxycitrate modulated locomotor function toward increased wire hang performance and reduced rotarod performance in healthy-fed mice, and improved locomotion in mice exposed to cardiotoxin-induced muscle atrophy. Our findings implicate ACLY and ACLY inhibitors in different aspects of aging and muscle regeneration.
Keywords: ACLY; health span; hydroxycitrate; lifespan; liver; muscle strength; tissue regeneration.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
VCG and AMM have filed a patent entitled “hidroxicitrato para la regeneración muscular.”
Figures





References
-
- Baardman, J. , Verberk, S. G. S. , van der Velden, S. , Gijbels, M. J. J. , van Roomen, C. P. P. A. , Sluimer, J. C. , Broos, J. Y. , Griffith, G. R. , Prange, K. H. M. , van Weeghel, M. , Lakbir, S. , Molenaar, D. , Meinster, E. , Neele, A. E. , Kooij, G. , de Vries, H. E. , Lutgens, E. , Wellen, K. E. , de Winther, M. P. J. , & Van den Bossche, J. (2020). Macrophage ATP citrate lyase deficiency stabilizes atherosclerotic plaques. Nature Communications, 11(1), 6296. 10.1038/s41467-020-20141-z - DOI - PMC - PubMed
-
- Birkenfeld, A. L. , Lee, H. Y. , Guebre‐Egziabher, F. , Alves, T. C. , Jurczak, M. J. , Jornayvaz, F. R. , Zhang, D. , Hsiao, J. J. , Martin‐Montalvo, A. , Fischer‐Rosinsky, A. , Spranger, J. , Pfeiffer, A. F. , Jordan, J. , Fromm, M. F. , König, J. , Lieske, S. , Carmean, C. M. , Frederick, D. W. , Weismann, D. , … Shulman, G. I. (2011). Deletion of the mammalian INDY homolog mimics aspects of dietary restriction and protects against adiposity and insulin resistance in mice. Cell Metabolism, 14(2), 184–195. 10.1016/j.cmet.2011.06.009 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

