Elimination of damaged mitochondria during UVB-induced senescence is orchestrated by NIX-dependent mitophagy
- PMID: 38761001
- PMCID: PMC11320349
- DOI: 10.1111/acel.14186
Elimination of damaged mitochondria during UVB-induced senescence is orchestrated by NIX-dependent mitophagy
Abstract
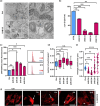
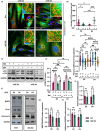
Skin aging is the result of two types of aging, "intrinsic aging" an inevitable consequence of physiologic and genetically determined changes and "extrinsic aging," which is dependent on external factors such as exposure to sunlight, smoking, and dietary habits. UVB causes skin injury through the generation of free radicals and other oxidative byproducts, also contributing to DNA damage. Appearance and accumulation of senescent cells in the skin are considered one of the hallmarks of aging in this tissue. Mitochondria play an important role for the development of cellular senescence, in particular stress-induced senescence of human cells. However, many aspects of mitochondrial physiology relevant to cellular senescence and extrinsic skin aging remain to be unraveled. Here, we demonstrate that mitochondria damaged by UVB irradiation of human dermal fibroblasts (HDF) are eliminated by NIX-dependent mitophagy and that this process is important for cell survival under these conditions. Additionally, UVB-irradiation of human dermal fibroblasts (HDF) induces the shedding of extracellular vesicles (EVs), and this process is significantly enhanced in UVB-irradiated NIX-depleted cells. Our findings establish NIX as the main mitophagy receptor in the process of UVB-induced senescence and suggest the release of EVs as an alternative mechanism of mitochondrial quality control in HDF.
Keywords: NIX; UVB; mitochondria; mitophagy; senescence; skin aging; vesicles.
© 2024 The Authors. Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that there are no competing interests associated with this manuscript.
Figures




References
-
- Anand, S. K. , Sharma, A. , Singh, N. , & Kakkar, P. (2020). Entrenching role of cell cycle checkpoints and autophagy for maintenance of genomic integrity. DNA Repair, 86, 102748. - PubMed
-
- Blume‐Peytavi, U. , Kottner, J. , Sterry, W. , Hodin, M. W. , Griffiths, T. W. , Watson, R. E. B. , Hay, R. J. , & Griffiths, C. E. M. (2016). Age‐associated skin conditions and diseases: Current perspectives and future options. Gerontologist, 56(Suppl 2), S230–S242. - PubMed
-
- Borghesan, M. , Fafián‐Labora, J. , Eleftheriadou, O. , Carpintero‐Fernández, P. , Paez‐Ribes, M. , Vizcay‐Barrena, G. , Swisa, A. , Kolodkin‐Gal, D. , Ximénez‐Embún, P. , Lowe, R. , Martín‐Martín, B. , Peinado, H. , Muñoz, J. , Fleck, R. A. , Dor, Y. , Ben‐Porath, I. , Vossenkamper, A. , Muñoz‐Espin, D. , & O'Loghlen, A. (2019). Small extracellular vesicles are key regulators of non‐cell autonomous intercellular communication in senescence via the interferon protein IFITM3. Cell Reports, 27, 3956–3971.e6. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

