Prognostic Impact of CYP2C19 Genotypes on Long-Term Clinical Outcomes in Older Patients After Percutaneous Coronary Intervention
- PMID: 38761068
- PMCID: PMC11179831
- DOI: 10.1161/JAHA.123.032248
Prognostic Impact of CYP2C19 Genotypes on Long-Term Clinical Outcomes in Older Patients After Percutaneous Coronary Intervention
Abstract
Background: Carriers of CYP2C19 loss-of-function alleles have increased adverse events after percutaneous coronary intervention, but limited data are available for older patients. We aimed to evaluate the prognostic impact of CYP2C19 genotypes on clinical outcomes in older patients after percutaneous coronary intervention.
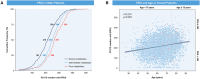
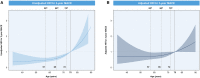
Methods and results: The study included 1201 older patients (aged ≥75 years) who underwent percutaneous coronary intervention and received clopidogrel-based dual antiplatelet therapy in South Korea. Patients were grouped on the basis of CYP2C19 genotypes. The primary outcome was 3-year major adverse cardiac events, defined as a composite of cardiac death, myocardial infarction, and stent thrombosis. Older patients were grouped into 3 groups: normal metabolizer (36.6%), intermediate metabolizer (48.1%), and poor metabolizer (15.2%). The occurrence of the primary outcome was significantly different among the groups (3.1, 7.0, and 6.2% in the normal metabolizer, intermediate metabolizer, and poor metabolizer groups, respectively; P=0.02). The incidence rate of all-cause death at 3 years was greater in the intermediate metabolizer and poor metabolizer groups (8.1% and 9.2%, respectively) compared with that in the normal metabolizer group (3.5%, P=0.03) without significant differences in major bleeding. In the multivariable analysis, the intermediate metabolizer and poor metabolizer groups were independent predictors of 3-year clinical outcomes.
Conclusions: In older patients, the presence of any CYP2C19 loss-of-function allele was found to be predictive of a higher incidence of major adverse cardiac events within 3 years following percutaneous coronary intervention. This finding suggests a need for further investigation into an optimal antiplatelet strategy for older patients.
Registration: URL: https://clinicaltrials.gov. Identifier: NCT04734028.
Keywords: aged; cytochrome P‐450 2C19; genetics; genotype; percutaneous coronary intervention; polymorphism.
Figures
References
-
- Caudle KE, Dunnenberger HM, Freimuth RR, Peterson JF, Burlison JD, Whirl‐Carrillo M, Scott SA, Rehm HL, Williams MS, Klein TE, et al. Standardizing terms for clinical pharmacogenetic test results: consensus terms from the clinical pharmacogenetics implementation consortium (CPIC). Genet Med. 2017;19:215–223. doi: 10.1038/gim.2016.87 - DOI - PMC - PubMed
-
- Simon T, Bhatt DL, Bergougnan L, Farenc C, Pearson K, Perrin L, Vicaut E, Lacreta F, Hurbin F, Dubar M. Genetic polymorphisms and the impact of a higher clopidogrel dose regimen on active metabolite exposure and antiplatelet response in healthy subjects. Clin Pharmacol Ther. 2011;90:287–295. doi: 10.1038/clpt.2011.127 - DOI - PubMed
-
- Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, et al. Reduced‐function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta‐analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543 - DOI - PMC - PubMed
-
- Pereira NL, Rihal C, Lennon R, Marcus G, Shrivastava S, Bell MR, So D, Geller N, Goodman SG, Hasan A, et al. Effect of CYP2C19 genotype on ischemic outcomes during Oral P2Y(12) inhibitor therapy: a meta‐analysis. JACC Cardiovasc Interv. 2021;14:739–750. doi: 10.1016/j.jcin.2021.01.024 - DOI - PMC - PubMed
-
- Lee CR, Luzum JA, Sangkuhl K, Gammal RS, Sabatine MS, Stein CM, Kisor DF, Limdi NA, Lee YM, Scott SA, et al. Clinical pharmacogenetics implementation consortium guideline for CYP2C19 genotype and clopidogrel therapy: 2022 update. Clin Pharmacol Ther. 2022;112:959–967. doi: 10.1002/cpt.2526 - DOI - PMC - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

