PDIA3 driven STAT3/PD-1 signaling promotes M2 TAM polarization and aggravates colorectal cancer progression
- PMID: 38761176
- PMCID: PMC11164521
- DOI: 10.18632/aging.205847
PDIA3 driven STAT3/PD-1 signaling promotes M2 TAM polarization and aggravates colorectal cancer progression
Abstract
Objective: This inquiry endeavors to delineate the influence of PDIA3 on tumor-associated macrophages within the realm of colorectal malignancies, whilst elucidating the intrinsic biochemical pathways.
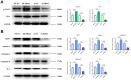
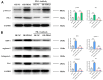
Method: Leveraging bioinformatics, we scrutinized the symbiosis between PDIA3, STAT3, and CD274. A xenograft model in immunodeficient murine served to assess PDIA3's impact on colorectal carcinogenesis. Further, Western blot analysis quantified the protein expression of PDIA3, p-STAT3, PD-1, XBP-1, assorted enzymes, and IL-6. Moreover, in vitro assays gauged SW480 cellular dynamics inclusive of migration, invasive potential, and proliferation.
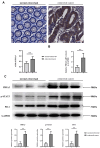
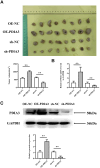
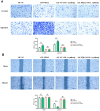
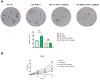
Results: Bioinformatics exploration exposed PDIA3's elevated presence in diverse cancers, with a marked expression in colorectal cancer, as per TCGA and GEO repositories. Correlative studies showed PDIA3 positively aligning with STAT3 and CD274, the latter also associated with monocyte-derived macrophages. Comparative analysis of colorectal neoplasms and normal colon samples unveiled heightened levels of PDIA3 markers which, when overexpressed in SW480 cells, escalated tumorigenicity and oncogenic behaviors, with a noted decrease upon PD-1 monoclonal antibody intervention.
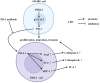
Conclusions: PDIA3 augments the M2 polarization of tumor-associated macrophages via modulation of the STAT3/PD-1 cascade, thus invigorating the tumorous proliferation and dissemination in colorectal cancer. Such revelations position PDIA3 as an auspicious target for PD-1 blockade therapeutics, offering a promising foundation for rectifying colorectal carcinoma.
Keywords: PDIA3; cancer microecology; colorectal neoplasm; immunological subterfuge; neoplastic therapy.
Conflict of interest statement
Figures

Similar articles
-
Downregulation of protein disulfide‑isomerase A3 expression inhibits cell proliferation and induces apoptosis through STAT3 signaling in hepatocellular carcinoma.Int J Oncol. 2019 Apr;54(4):1409-1421. doi: 10.3892/ijo.2019.4710. Epub 2019 Feb 4. Int J Oncol. 2019. PMID: 30720090
-
CPEB3 inhibits epithelial-mesenchymal transition by disrupting the crosstalk between colorectal cancer cells and tumor-associated macrophages via IL-6R/STAT3 signaling.J Exp Clin Cancer Res. 2020 Jul 11;39(1):132. doi: 10.1186/s13046-020-01637-4. J Exp Clin Cancer Res. 2020. PMID: 32653013 Free PMC article.
-
PD-1 regulates the anti-tumor immune function of macrophages through JAK2-STAT3 signaling pathway in colorectal cancer tumor microenvironment.J Transl Med. 2025 May 2;23(1):502. doi: 10.1186/s12967-025-06469-4. J Transl Med. 2025. PMID: 40317043 Free PMC article.
-
KLHDC8A Regulates M1/M2 Macrophage Polarization Through the PD-1/STAT3 Pathway to Promote Papillary Thyroid Cancer Development.Discov Med. 2024 Sep;36(188):1858-1868. doi: 10.24976/Discov.Med.202436188.172. Discov Med. 2024. PMID: 39327249
-
Converged Rab37/IL-6 trafficking and STAT3/PD-1 transcription axes elicit an immunosuppressive lung tumor microenvironment.Theranostics. 2021 May 12;11(14):7029-7044. doi: 10.7150/thno.60040. eCollection 2021. Theranostics. 2021. PMID: 34093869 Free PMC article.
Cited by
-
Identification of novel diagnostic biomarkers associated with liver metastasis in colon adenocarcinoma by machine learning.Discov Oncol. 2024 Oct 10;15(1):542. doi: 10.1007/s12672-024-01398-y. Discov Oncol. 2024. PMID: 39390264 Free PMC article.
-
Combination of low-intensity pulsed ultrasound irradiating immune organs with immune checkpoint blockade augments systemic anti-tumor immunity on low tumor burden 4T-1 breast cancer.Cancer Immunol Immunother. 2025 Aug 6;74(9):281. doi: 10.1007/s00262-025-04137-6. Cancer Immunol Immunother. 2025. PMID: 40768050 Free PMC article.
References
-
- Gobert AP, Latour YL, Asim M, Barry DP, Allaman MM, Finley JL, Smith TM, McNamara KM, Singh K, Sierra JC, Delgado AG, Luis PB, Schneider C, et al.. Protective Role of Spermidine in Colitis and Colon Carcinogenesis. Gastroenterology. 2022; 162:813–27.e8. 10.1053/j.gastro.2021.11.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

