Validation of a risk score to differentiate autoimmune and viral encephalitis: a Nationwide Cohort Study in Denmark
- PMID: 38761191
- PMCID: PMC11319475
- DOI: 10.1007/s00415-024-12392-3
Validation of a risk score to differentiate autoimmune and viral encephalitis: a Nationwide Cohort Study in Denmark
Abstract
Background: A score to differentiate autoimmune (AE) and viral encephalitis (VE) early upon admission has recently been developed but needed external validation. The objective of this study was to evaluate the performance of the score in a larger and more diagnostically diverse patient cohort.
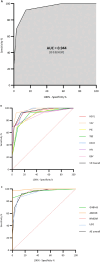
Methods: We conducted a retrospective nationwide and population-based cohort study including all adults with encephalitis of definite viral (2015-2022) or autoimmune aetiology (2009-2022) in Denmark. Variables included in the score-model were extracted from patient records and individual risk scores were assessed. The performance of the score was assessed by receiver-operating characteristics (ROC) curve analyses and calculation of the area under the curve (AUC).
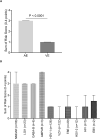
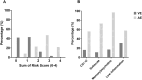
Results: A total of 496 patients with encephalitis [AE n = 90, VE n = 287 and presumed infectious encephalitis (PIE) n = 119] were included in the study. The score was highly accurate in predicting cases of AE reaching an AUC of 0.94 (95% CI 0.92-0.97). Having a score ≥ 3 predicted AE with a PPV of 87% and an NPV of 91%. The risk score was found to perform well across aetiological subgroups and applied to the PIE cohort resulted in an AUC of 0.88 (95% CI 0.84-0.93).
Conclusion: The excellent performance of the score as reported in the development study was confirmed in this significantly larger and more diverse cohort of patients with encephalitis in Denmark. These results should prompt further prospective testing with wider inclusion criteria.
Keywords: Autoimmune encephalitis; Encephalitis; Risk score; Validation; Viral encephalitis.
© 2024. The Author(s).
Conflict of interest statement
AML reports speakers’ honorarium/travel grants/advisory board activity and unrestricted grant from Gilead, speakers honorarium/travel grants from GSK, and speaker’s honorarium/advisory board activity from Pfizer outside this work.
Figures
Similar articles
-
Development of a prediction model integrating PD-1 and ICOS for early differential diagnosis between autoimmune and viral encephalitis.Front Immunol. 2025 Apr 25;16:1550963. doi: 10.3389/fimmu.2025.1550963. eCollection 2025. Front Immunol. 2025. PMID: 40352922 Free PMC article.
-
Development and Validation of a Risk Score to Differentiate Viral and Autoimmune Encephalitis in Adults.Clin Infect Dis. 2023 Feb 8;76(3):e1294-e1301. doi: 10.1093/cid/ciac711. Clin Infect Dis. 2023. PMID: 36053949
-
Differentiation between viral and autoimmune limbic encephalitis: a prospective cohort study with development and validation of a diagnostic model.J Neurol. 2024 Aug;271(8):5301-5311. doi: 10.1007/s00415-024-12468-0. Epub 2024 Jun 11. J Neurol. 2024. PMID: 38858284
-
Performance of the clinical assessment scale for autoimmune encephalitis in a pediatric autoimmune encephalitis cohort.Front Immunol. 2022 Oct 14;13:915352. doi: 10.3389/fimmu.2022.915352. eCollection 2022. Front Immunol. 2022. PMID: 36311740 Free PMC article. Review.
-
Evaluation of the clinical assessment scale for autoimmune encephalitis (CASE) in a retrospective cohort and a systematic review.Neurol Sci. 2024 Nov;45(11):5423-5428. doi: 10.1007/s10072-024-07642-1. Epub 2024 Jun 11. Neurol Sci. 2024. PMID: 38862652 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

