Quantification of age-related changes in the structure and mechanical function of skin with multiscale imaging
- PMID: 38761286
- PMCID: PMC11336155
- DOI: 10.1007/s11357-024-01199-9
Quantification of age-related changes in the structure and mechanical function of skin with multiscale imaging
Abstract
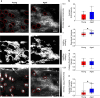
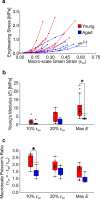
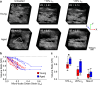
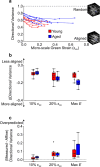
The mechanical properties of skin change during aging but the relationships between structure and mechanical function remain poorly understood. Previous work has shown that young skin exhibits a substantial decrease in tissue volume, a large macro-scale Poisson's ratio, and an increase in micro-scale collagen fiber alignment during mechanical stretch. In this study, label-free multiphoton microscopy was used to quantify how the microstructure and fiber kinematics of aged mouse skin affect its mechanical function. In an unloaded state, aged skin was found to have less collagen alignment and more non-enzymatic collagen fiber crosslinks. Skin samples were then loaded in uniaxial tension and aged skin exhibited a lower mechanical stiffness compared to young skin. Aged tissue also demonstrated less volume reduction and a lower macro-scale Poisson's ratio at 10% uniaxial strain, but not at 20% strain. The magnitude of 3D fiber realignment in the direction of loading was not different between age groups, and the amount of realignment in young and aged skin was less than expected based on theoretical fiber kinematics affine to the local deformation. These findings provide key insights on how the collagen fiber microstructure changes with age, and how those changes affect the mechanical function of skin, findings which may help guide wound healing or anti-aging treatments.
Keywords: Biomechanics; Intrinsic Aging; Multi-scale; Multiphoton Microscopy; Second Harmonic Generation; Skin.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures

Similar articles
-
Three-Dimensional Quantification of Collagen Microstructure During Tensile Mechanical Loading of Skin.Front Bioeng Biotechnol. 2021 Mar 3;9:642866. doi: 10.3389/fbioe.2021.642866. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33748088 Free PMC article.
-
Multiscale Computational Model Predicts Mouse Skin Kinematics Under Tensile Loading.J Biomech Eng. 2022 Apr 1;144(4):041008. doi: 10.1115/1.4052887. J Biomech Eng. 2022. PMID: 34729595 Free PMC article.
-
Biomechanical and biochemical changes in murine skin during development and aging.Acta Biomater. 2024 Sep 15;186:316-329. doi: 10.1016/j.actbio.2024.07.013. Epub 2024 Jul 14. Acta Biomater. 2024. PMID: 39009208
-
How aging impacts skin biomechanics: a multiscale study in mice.Sci Rep. 2017 Oct 23;7(1):13750. doi: 10.1038/s41598-017-13150-4. Sci Rep. 2017. PMID: 29061975 Free PMC article.
-
Skin Structure-Function Relationships and the Wound Healing Response to Intrinsic Aging.Adv Wound Care (New Rochelle). 2020 Mar 1;9(3):127-143. doi: 10.1089/wound.2019.1021. Epub 2020 Jan 24. Adv Wound Care (New Rochelle). 2020. PMID: 31993254 Free PMC article. Review.
Cited by
-
Mechanical Models of Collagen Networks for Understanding Changes in the Failure Properties of Aging Skin.J Biomech Eng. 2024 Jul 1;146(7):071002. doi: 10.1115/1.4064406. J Biomech Eng. 2024. PMID: 38183223 Free PMC article.
References
-
- Fisher GJ, Quan T, Purohit T, Shao Y, Cho MK, He T, Varani J, Kang S, Voorhees JJ. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am J Pathol. 2009;174:101–14. 10.2353/ajpath.2009.080599. 10.2353/ajpath.2009.080599 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

