Comparison of clinical outcomes in hospitalized patients with COVID-19 or non-COVID-19 community-acquired pneumonia in a prospective observational cohort study
- PMID: 38761325
- PMCID: PMC11621138
- DOI: 10.1007/s15010-024-02292-z
Comparison of clinical outcomes in hospitalized patients with COVID-19 or non-COVID-19 community-acquired pneumonia in a prospective observational cohort study
Abstract
Purpose: Coronavirus disease 2019 (COVID-19) and non-COVID-19 community-acquired pneumonia (NC-CAP) often result in hospitalization with considerable risks of mortality, ICU treatment, and long-term morbidity. A comparative analysis of clinical outcomes in COVID-19 CAP (C-CAP) and NC-CAP may improve clinical management.
Methods: Using prospectively collected CAPNETZ study data (January 2017 to June 2021, 35 study centers), we conducted a comprehensive analysis of clinical outcomes including in-hospital death, ICU treatment, length of hospital stay (LOHS), 180-day survival, and post-discharge re-hospitalization rate. Logistic regression models were used to examine group differences between C-CAP and NC-CAP patients and associations with patient demography, recruitment period, comorbidity, and treatment.
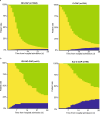
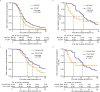
Results: Among 1368 patients (C-CAP: n = 344; NC-CAP: n = 1024), C-CAP showed elevated adjusted probabilities for in-hospital death (aOR 4.48 [95% CI 2.38-8.53]) and ICU treatment (aOR 8.08 [95% CI 5.31-12.52]) compared to NC-CAP. C-CAP patients were at increased risk of LOHS over seven days (aOR 1.88 [95% CI 1.47-2.42]). Although ICU patients had similar in-hospital mortality risk, C-CAP was associated with length of ICU stay over seven days (aOR 3.59 [95% CI 1.65-8.38]). Recruitment period influenced outcomes in C-CAP but not in NC-CAP. During follow-up, C-CAP was linked to a reduced risk of re-hospitalization and mortality post-discharge (aOR 0.43 [95% CI 0.27-0.70]).
Conclusion: Distinct clinical trajectories of C-CAP and NC-CAP underscore the need for adapted management to avoid acute and long-term morbidity and mortality amid the evolving landscape of CAP pathogens.
Keywords: COVID-19; Community-acquired pneumonia; Observational cohort study; SARS-CoV-2.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: H.-J. M. reports a personal fee from Astra Zeneca for a lecture. G. R. reports personal fees from Astra Zeneca, Atriva, Boehringer Ingelheim, GSK, Insmed, MSD, Sanofi, Novartis, and Pfizer for consultancy during advisory board meetings and personal fees from Astra Zeneca, Berlin Chemie, BMS, Boehringer Ingelheim, Chiesi, Essex Pharma, Grifols, GSK, Insmed, MSD, Roche, Sanofi, Solvay, Takeda, Novartis, Pfizer, and Vertex for lectures. M. W. received funding for research, lectures, or advisory from Alexion, Aptarion, Astra Zeneca, Bayer Health Care, Biotest, Boehringer Ingelheim, Chiesi, Glaxo Smith Kline, Insmed, Novartis, Pantherna, Pfizer, Teva, and Vaxxilon. T. Z. received funding for research from Bundesministerium für Bildung und Forschung, Else Kröner Fresenius Stiftung and Gesellschaft für Internationale Zusammenarbeit.
Figures

References
-
- Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Swartz S, Fullman N, Mosser J, Thompson RL, Reiner RC, Abajobir A. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet Infect Dis. 2017;17:1133–61. 10.1016/S1473-3099(17)30396-1. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

