Transient caspase-mediated activation of caspase-activated DNase causes DNA damage required for phagocytic macrophage differentiation
- PMID: 38761374
- PMCID: PMC7617294
- DOI: 10.1016/j.celrep.2024.114251
Transient caspase-mediated activation of caspase-activated DNase causes DNA damage required for phagocytic macrophage differentiation
Abstract
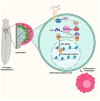
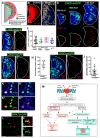
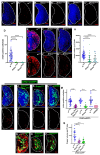
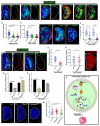
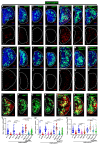
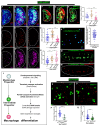
Phagocytic macrophages are crucial for innate immunity and tissue homeostasis. Most tissue-resident macrophages develop from embryonic precursors that populate every organ before birth to lifelong self-renew. However, the mechanisms for versatile macrophage differentiation remain unknown. Here, we use in vivo genetic and cell biological analysis of the Drosophila larval hematopoietic organ, the lymph gland that produces macrophages. We show that the developmentally regulated transient activation of caspase-activated DNase (CAD)-mediated DNA strand breaks in intermediate progenitors is essential for macrophage differentiation. Insulin receptor-mediated PI3K/Akt signaling regulates the apoptosis signal-regulating kinase 1 (Ask1)/c-Jun kinase (JNK) axis to control sublethal levels of caspase activation, causing DNA strand breaks during macrophage development. Furthermore, caspase activity is also required for embryonic-origin macrophage development and efficient phagocytosis. Our study provides insights into developmental signaling and CAD-mediated DNA strand breaks associated with multifunctional and heterogeneous macrophage differentiation.
Keywords: CAD/ICAD; CP: Immunology; Draper; Drosophila; caspase; hematopoiesis; intermediate progenitor cells; lymph gland; myeloid progenitors; γH2Av.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Comment in
-
Destruction as a creative process: CAD-induced DNA strand breaks promote macrophage differentiation.Cell Rep. 2024 Oct 22;43(10):114816. doi: 10.1016/j.celrep.2024.114816. Epub 2024 Sep 25. Cell Rep. 2024. PMID: 39325618
References
-
- Gosselin D, Link VM, Romanoski CE, Fonseca GJ, Eichenfield DZ, Spann NJ, Stender JD, Chun HB, Garner H, Geissmann F, Glass CK. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell. 2014;159:1327–1340. doi: 10.1016/j.cell.2014.11.023. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

