Clostridioides difficile toxin B subverts germinal center and antibody recall responses by stimulating a drug-treatable CXCR4-dependent mechanism
- PMID: 38761377
- PMCID: PMC11210377
- DOI: 10.1016/j.celrep.2024.114245
Clostridioides difficile toxin B subverts germinal center and antibody recall responses by stimulating a drug-treatable CXCR4-dependent mechanism
Abstract
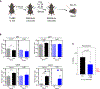
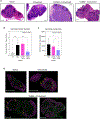
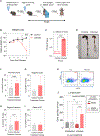
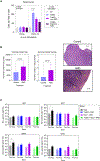
Recurrent Clostridioides difficile infection (CDI) results in significant morbidity and mortality. We previously established that CDI in mice does not protect against reinfection and is associated with poor pathogen-specific B cell memory (Bmem), recapitulating our observations with human Bmem. Here, we demonstrate that the secreted toxin TcdB2 is responsible for subversion of Bmem responses. TcdB2 from an endemic C. difficile strain delayed immunoglobulin G (IgG) class switch following vaccination, attenuated IgG recall to a vaccine booster, and prevented germinal center formation. The mechanism of TcdB2 action included increased B cell CXCR4 expression and responsiveness to its ligand CXCL12, accounting for altered cell migration and a failure of germinal center-dependent Bmem. These results were reproduced in a C. difficile infection model, and a US Food and Drug Administration (FDA)-approved CXCR4-blocking drug rescued germinal center formation. We therefore provide mechanistic insights into C. difficile-associated pathogenesis and illuminate a target for clinical intervention to limit recurrent disease.
Keywords: B lymphocyte; C. difficile; CP: Immunology; CP: Microbiology; CXCR4; antibody; chemotaxis; germinal center; toxin.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests K.M.N., M.L.L., and J.D.B. are listed as inventors on provisional US patent OKLA.P0023US.P1 filed on 09/13/23, which is related to the work described here.
Figures



Similar articles
-
Memory B Cells Encode Neutralizing Antibody Specific for Toxin B from the Clostridium difficile Strains VPI 10463 and NAP1/BI/027 but with Superior Neutralization of VPI 10463 Toxin B.Infect Immun. 2015 Oct 26;84(1):194-204. doi: 10.1128/IAI.00011-15. Print 2016 Jan. Infect Immun. 2015. PMID: 26502913 Free PMC article.
-
Circulating antibody and memory B-Cell responses to C. difficile toxins A and B in patients with C. difficile-associated diarrhoea, inflammatory bowel disease and cystic fibrosis.PLoS One. 2013 Sep 10;8(9):e74452. doi: 10.1371/journal.pone.0074452. eCollection 2013. PLoS One. 2013. PMID: 24058568 Free PMC article.
-
Clostridioides difficile Infection Induces an Inferior IgG Response to That Induced by Immunization and Is Associated with a Lack of T Follicular Helper Cell and Memory B Cell Expansion.Infect Immun. 2020 Feb 20;88(3):e00829-19. doi: 10.1128/IAI.00829-19. Print 2020 Feb 20. Infect Immun. 2020. PMID: 31871095 Free PMC article.
-
The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection.Mol Immunol. 2015 Feb;63(2):193-202. doi: 10.1016/j.molimm.2014.09.005. Epub 2014 Sep 18. Mol Immunol. 2015. PMID: 25242213 Free PMC article. Review.
-
The role of toxins in Clostridium difficile infection.FEMS Microbiol Rev. 2017 Nov 1;41(6):723-750. doi: 10.1093/femsre/fux048. FEMS Microbiol Rev. 2017. PMID: 29048477 Free PMC article. Review.
Cited by
-
Clostridioides difficile toxin A and toxin B inhibit toxin-specific adaptive immune responses through glucosyltransferase-dependent activity.bioRxiv [Preprint]. 2025 Aug 2:2025.07.30.667646. doi: 10.1101/2025.07.30.667646. bioRxiv. 2025. PMID: 40766602 Free PMC article. Preprint.
-
Enhanced immunogenicity of a Clostridioides difficile TcdB vaccine adjuvanted with a synthetic dual-TLR ligand adjuvant.NPJ Vaccines. 2025 Feb 18;10(1):33. doi: 10.1038/s41541-025-01075-3. NPJ Vaccines. 2025. PMID: 39966390 Free PMC article.
-
Protocol to measure the impact of Clostridioides difficile toxins on antibody responses using ELISA, ELISPOT, and toxin-neutralization assays.STAR Protoc. 2025 Jun 20;6(2):103754. doi: 10.1016/j.xpro.2025.103754. Epub 2025 Apr 8. STAR Protoc. 2025. PMID: 40202837 Free PMC article.
-
A multivalent mRNA-LNP vaccine protects against Clostridioides difficile infection.Science. 2024 Oct 4;386(6717):69-75. doi: 10.1126/science.adn4955. Epub 2024 Oct 3. Science. 2024. PMID: 39361752 Free PMC article.
-
The interplay between host immunity and Clostridioides difficile infection.mBio. 2025 Aug 13;16(8):e0356224. doi: 10.1128/mbio.03562-24. Epub 2025 Jul 1. mBio. 2025. PMID: 40590563 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

