Distinct immunometabolic signatures in circulating immune cells define disease outcome in acute-on-chronic liver failure
- PMID: 38761406
- PMCID: PMC11737128
- DOI: 10.1097/HEP.0000000000000907
Distinct immunometabolic signatures in circulating immune cells define disease outcome in acute-on-chronic liver failure
Abstract
Background and aims: Acute-on-chronic liver failure (ACLF) is a complication of cirrhosis characterized by multiple organ failure and high short-term mortality. The pathophysiology of ACLF involves elevated systemic inflammation leading to organ failure, along with immune dysfunction that heightens susceptibility to bacterial infections. However, it is unclear how these aspects are associated with recovery and nonrecovery in ACLF.
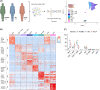
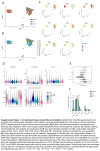
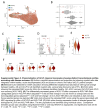
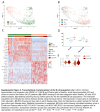
Approach and results: Here, we mapped the single-cell transcriptome of circulating immune cells from patients with ACLF and acute decompensated (AD) cirrhosis and healthy individuals. We further interrogate how these findings, as well as immunometabolic and functional profiles, associate with ACLF-recovery (ACLF-R) or nonrecovery (ACLF-NR). Our analysis unveiled 2 distinct states of classical monocytes (cMons). Hereto, ACLF-R cMons were characterized by transcripts associated with immune and stress tolerance, including anti-inflammatory genes such as RETN and LGALS1 . Additional metabolomic and functional validation experiments implicated an elevated oxidative phosphorylation metabolic program as well as an impaired ACLF-R cMon functionality. Interestingly, we observed a common stress-induced tolerant state, oxidative phosphorylation program, and blunted activation among lymphoid populations in patients with ACLF-R. Conversely, ACLF-NR cMon featured elevated expression of inflammatory and stress response genes such as VIM , LGALS2 , and TREM1 , along with blunted metabolic activity and increased functionality.
Conclusions: This study identifies distinct immunometabolic cellular states that contribute to disease outcomes in patients with ACLF. Our findings provide valuable insights into the pathogenesis of ACLF, shedding light on factors driving either recovery or nonrecovery phenotypes, which may be harnessed as potential therapeutic targets in the future.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
Hannah van Malenstein consults for Boston Scientific. Vicente Arroyo consults for Grifols. The remaining authors have no conflicts to report.
Figures







References
-
- Arroyo V, Moreau R, Kamath PS, Jalan R, Ginès P, Nevens F, et al. . Acute-on-chronic liver failure in cirrhosis. Nat Rev Dis Primers. 2016;2:16041. - PubMed
-
- Arroyo V, Moreau R, Jalan R. Acute-on-chronic liver failure. N Engl J Med. 2020;382:2137–2145. - PubMed
-
- Meersseman P, Langouche L, du Plessis J, Korf H, Mekeirele M, Laleman W, et al. . The intensive care unit course and outcome in acute-on-chronic liver failure are comparable to other populations. J Hepatol. 2018;69:803–809. - PubMed
-
- Van der Merwe S, Chokshi S, Bernsmeier C, Albillos A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J Hepatol. 2021;75:S82–S100. - PubMed
-
- Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2021;19:112–134. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

