Omega-3 fatty acid diglyceride emulsions as a novel injectable acute therapeutic in neonatal hypoxic-ischemic brain injury
- PMID: 38761420
- PMCID: PMC11156760
- DOI: 10.1016/j.biopha.2024.116749
Omega-3 fatty acid diglyceride emulsions as a novel injectable acute therapeutic in neonatal hypoxic-ischemic brain injury
Abstract
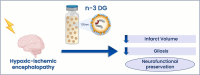
Hypoxic-ischemic encephalopathy (HIE), resulting from a lack of blood flow and oxygen before or during newborn delivery, is a leading cause of cerebral palsy and neurological disability in children. Therapeutic hypothermia (TH), the current standard of care in HIE, is only beneficial in 1 of 7-8 cases. Therefore, there is a critical need for more efficient treatments. We have previously reported that omega-3 (n-3) fatty acids (FA) carried by triglyceride (TG) lipid emulsions provide neuroprotection after experimental hypoxic-ischemic (HI) injury in neonatal mice. Herein, we propose a novel acute therapeutic approach using an n-3 diglyceride (DG) lipid emulsions. Importantly, n-3 DG preparations had much smaller particle size compared to commercially available or lab-made n-3 TG emulsions. We showed that n-3 DG molecules have the advantage of incorporating at substantially higher levels than n-3 TG into an in vitro model of phospholipid membranes. We also observed that n-3 DG after parenteral administration in neonatal mice reaches the bloodstream more rapidly than n-3 TG. Using neonatal HI brain injury models in mice and rats, we found that n-3 DG emulsions provide superior neuroprotection than n-3 TG emulsions or TH in decreasing brain infarct size. Additionally, we found that n-3 DGs attenuate microgliosis and astrogliosis. Thus, n-3 DG emulsions are a superior, promising, and novel therapy for treating HIE.
Keywords: diglycerides; gliosis; hypoxic-ischemic encephalopathy; lipid emulsion; neuroprotection; omega-3 fatty acids.
Copyright © 2024 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest Richard J Deckelbaum is a founding scientist and scientific advisory board member of DeckTherapeutics Inc., a company that plans to use novel n-3 lipid emulsions to prevent tissue death after ischemic brain injury. Hylde Zirpoli is a scientific advisory board member of DeckTherapeutics Inc. DeckTherapeutics Inc. had no inputs or roles in the experimental design, data analysis and funding of this paper. The other authors declare no conflict of interest.
Figures






Similar articles
-
N-3 fatty acid rich triglyceride emulsions are neuroprotective after cerebral hypoxic-ischemic injury in neonatal mice.PLoS One. 2013;8(2):e56233. doi: 10.1371/journal.pone.0056233. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437099 Free PMC article.
-
Dietary LPC-Bound n-3 LCPUFA Protects against Neonatal Brain Injury in Mice but Does Not Enhance Stem Cell Therapy.Nutrients. 2024 Jul 12;16(14):2252. doi: 10.3390/nu16142252. Nutrients. 2024. PMID: 39064695 Free PMC article.
-
Glucocorticoids Protect Neonatal Rat Brain in Model of Hypoxic-Ischemic Encephalopathy (HIE).Int J Mol Sci. 2016 Dec 22;18(1):17. doi: 10.3390/ijms18010017. Int J Mol Sci. 2016. PMID: 28025500 Free PMC article.
-
A Review of Plant Extracts and Plant-Derived Natural Compounds in the Prevention/Treatment of Neonatal Hypoxic-Ischemic Brain Injury.Int J Mol Sci. 2021 Jan 15;22(2):833. doi: 10.3390/ijms22020833. Int J Mol Sci. 2021. PMID: 33467663 Free PMC article. Review.
-
Novel Neuroprotective Agents to Treat Neonatal Hypoxic-Ischemic Encephalopathy: Inter-Alpha Inhibitor Proteins.Int J Mol Sci. 2020 Dec 2;21(23):9193. doi: 10.3390/ijms21239193. Int J Mol Sci. 2020. PMID: 33276548 Free PMC article. Review.
Cited by
-
Omega-3 Fatty Acids and Traumatic Injury in the Adult and Immature Brain.Nutrients. 2024 Nov 30;16(23):4175. doi: 10.3390/nu16234175. Nutrients. 2024. PMID: 39683568 Free PMC article. Review.
-
Exploring the landscape of Lipid Nanoparticles (LNPs): A comprehensive review of LNPs types and biological sources of lipids.Int J Pharm X. 2024 Nov 18;8:100305. doi: 10.1016/j.ijpx.2024.100305. eCollection 2024 Dec. Int J Pharm X. 2024. PMID: 39669003 Free PMC article. Review.
References
-
- Bonifacio S.L., Hutson S. The term newborn: evaluation for hypoxic-ischemic encephalopathy. Clin. Perinatol. 2021;48(3):681–695. - PubMed
-
- Amer Y.S., Anabrees J., Abdelmawla M., Abdalgader A., Almazroei A., Alhifzi I., AlOnazi A.H., Sabr Y., Hneiny L., El-Malky A., Alshalawi A., Alayoubi A., Chaudhry I.A., Elkhateeb O. Clinical practice guidelines for neonatal hypoxic-ischemic encephalopathy: a systematic review using the appraisal of guidelines for research and evaluation (AGREE) II instrument. Front Pedia. 2023;11:1092578. - PMC - PubMed
-
- Davidson J.O., Gonzalez F., Gressens P., Gunn A.J. Update on mechanisms of the pathophysiology of neonatal encephalopathy. Semin Fetal Neonatal Med. 2021;26(5) - PubMed
-
- Greco P., Nencini G., Piva I., Scioscia M., Volta C.A., Spadaro S., Neri M., Bonaccorsi G., Greco F., Cocco I., Sorrentino F., D'Antonio F., Nappi L. Pathophysiology of hypoxic-ischemic encephalopathy: a review of the past and a view on the future. Acta Neurol. Belg. 2020;120(2):277–288. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

