Effectiveness and safety of a novel, collapsible pessary for management of pelvic organ prolapse
- PMID: 38761837
- PMCID: PMC11283992
- DOI: 10.1016/j.ajog.2024.05.009
Effectiveness and safety of a novel, collapsible pessary for management of pelvic organ prolapse
Abstract
Background: Pessaries are an effective treatment for pelvic organ prolapse, yet currently available pessaries can cause discomfort during removal and insertion. An early feasibility trial of an investigational, collapsible pessary previously demonstrated mechanical feasibility during a brief 15-minute office trial. Longer-term, patient-centered safety and efficacy data are needed.
Objective: This study aimed to assess the effectiveness and safety of the investigational vaginal pessary for pelvic organ prolapse at 3 months.
Study design: This was a prospective, 7-center, open-label equivalence study with participants serving as their own controls. Subjects were current users of a Gellhorn or ring pessary with ≥stage 2 prolapse. Subjective and objective data were collected at baseline for 1 month while subjects used their current pessary. Data were then collected throughout a 3-month treatment phase with the study pessary. The primary outcome was change in Pelvic Floor Distress Inventory-20 score. Secondary outcome measures included objective assessment of prolapse support, changes in the Pelvic Floor Impact Questionnaire-7, and pain with insertion and removal, measured using a visual analog scale. Data from subjects fitted with the study pessary were analyzed using an intention-to-treat approach, and those who dropped out were assigned scores at the upper limit of the predefined equivalence limits. Secondary per protocol analyses included subjects who completed treatment. The study was powered to 80% with a minimal important change equivalence limit of 18.3 points on the Pelvic Floor Distress Inventory-20 scale. Square root transformations were used for nonparametric data, and P values were adjusted for multiple comparisons.
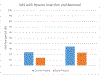
Results: A total of 78 subjects were enrolled, however, 16 withdrew before study pessary placement. The study pessary was fitted in 62 subjects (50 ring and 12 Gellhorn pessary users), and 48 (62%) completed the 3-month intervention. The change in Pelvic Floor Distress Inventory-20 scores at 3 months demonstrated equivalence when compared with the subjects' baseline scores (mean difference, -3.96 [improvement]; 90% confidence interval, -11.99 to 4.08; P=.002). Among those completing study, the Pelvic Floor Distress Inventory-20 scores, equivalence was not demonstrated and scores favored the study pessary (mean difference, -10.45; 90% confidence interval, -20.35 to 0.54; P=.095). Secondary outcomes included objective measures of support, which were similar (mean difference: Ba, 0.54 cm; Bp, 0.04 cm, favoring study pessary; improvement in mean Pelvic Floor Impact Questionnaire-7 scores for those who completed the trial: before, 32.23; after, 16.86; P=.019), and pain with insertion and removal, which was lower with the study pessary than with the subject's own pessary (mean difference visual analog scale score insertion, 9.91 mm; P=.019; removal, 11.23 mm; P=.019). No serious adverse events related to the pessary were reported.
Conclusion: Equivalence was demonstrated in the primary outcome of the study pessary when compared with current, noncollapsible pessaries in terms of change in severity and bother of pelvic floor symptoms. Among participants who completed the trial, the Pelvic Floor Impact Questionnaire-7 improved with study pessary use and change in Pelvic Floor Distress Inventory-20 scores were nonequivalent, favoring the study pessary. Subjects reported significantly lower pain scores with both pessary insertion and removal with the novel collapsible pessary when compared with their standard pessary.
Keywords: apical support; cystocele; pelvic organ prolapse; pessary; treatment.
Copyright © 2024 Elsevier Inc. All rights reserved.
Figures



References
-
- Samuelsson EC, Victor FT, Tibblin G, Svärdsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180(2 Pt 1):299–305. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

