Emerging targets in lipid metabolism for cancer therapy
- PMID: 38762377
- PMCID: PMC11162322
- DOI: 10.1016/j.tips.2024.04.007
Emerging targets in lipid metabolism for cancer therapy
Abstract
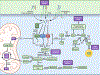
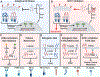
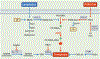
Cancer cells perturb lipid metabolic pathways for a variety of pro-tumorigenic functions, and deregulated cellular metabolism is a hallmark of cancer cells. Although alterations in lipid metabolism in cancer cells have been appreciated for over 20 years, there are no FDA-approved cancer treatments that target lipid-related pathways. Recent advances pertaining to cancer cell fatty acid synthesis (FAS), desaturation, and uptake, microenvironmental and dietary lipids, and lipid metabolism of tumor-infiltrating immune cells have illuminated promising clinical applications for targeting lipid metabolism. This review highlights emerging pathways and targets for tumor lipid metabolism that may soon impact clinical treatment.
Keywords: cancer metabolism; fatty acids; lipid metabolism.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests The authors have no competing interests to declare.
Figures

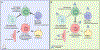
References
-
- Menendez JA and Lupu R (2007) Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer 7, 763–777 - PubMed
-
- Ookhtens M, et al. (1984) Liver and adipose tissue contributions to newly formed fatty acids in an ascites tumor. Am J Physiol 247, R146–153 - PubMed
-
- Scott JS, et al. (2022) Monounsaturated Fatty Acids: Key Regulators of Cell Viability and Intracellular Signaling in Cancer. Mol Cancer Res 20, 1354–1364 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

