Dynamic radiological features predict pathological response after neoadjuvant immunochemotherapy in esophageal squamous cell carcinoma
- PMID: 38762454
- PMCID: PMC11102630
- DOI: 10.1186/s12967-024-05291-8
Dynamic radiological features predict pathological response after neoadjuvant immunochemotherapy in esophageal squamous cell carcinoma
Abstract
Background: Neoadjuvant immunochemotherapy (NICT) plus esophagectomy has emerged as a promising treatment option for locally advanced esophageal squamous cell carcinoma (LA-ESCC). Pathologic complete response (pCR) is a key indicator associated with great efficacy and overall survival (OS). However, there are insufficient indicators for the reliable assessment of pCR.
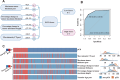
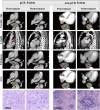
Methods: 192 patients with LA-ESCC treated with NICT from December 2019 to October 2023 were recruited. According to pCR status, patients were categorized into pCR group (22.92%) and non-pCR group (77.08%). Radiological features of pretreatment and preoperative CT images were extracted. Logistic and COX regressions were trained to predict pathological response and prognosis, respectively.
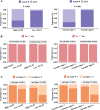
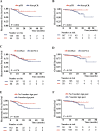
Results: Four of the selected radiological features were combined to construct an ESCC preoperative imaging score (ECPI-Score). Logistic models revealed independent associations of ECPI-Score and vascular sign with pCR, with AUC of 0.918 in the training set and 0.862 in the validation set, respectively. After grouping by ECPI-Score, a higher proportion of pCR was observed among the high-ECPI group and negative vascular sign. Kaplan Meier analysis demonstrated that recurrence-free survival (RFS) with negative vascular sign was significantly better than those with positive (P = 0.038), but not for OS (P = 0.310).
Conclusions: This study demonstrates dynamic radiological features are independent predictors of pCR for LA-ESCC treated with NICT. It will guide clinicians to make accurate treatment plans.
Trial registration: ClinicalTrials.gov NCC201908A03.
Keywords: Computed tomography; Esophageal cancer; Neoadjuvant PD-1 blockade; Pathological complete response.
© 2024. The Author(s).
Conflict of interest statement
The authors report there are no competing interests to declare.
Figures


Similar articles
-
CT-based delta-radiomics for predicting pathological response to neoadjuvant immunochemotherapy in esophageal squamous cell carcinoma: a multicenter study.BMC Med Imaging. 2024 Dec 3;24(1):329. doi: 10.1186/s12880-024-01503-1. BMC Med Imaging. 2024. PMID: 39627736 Free PMC article.
-
Voxel-level radiomics and deep learning for predicting pathologic complete response in esophageal squamous cell carcinoma after neoadjuvant immunotherapy and chemotherapy.J Immunother Cancer. 2025 Mar 15;13(3):e011149. doi: 10.1136/jitc-2024-011149. J Immunother Cancer. 2025. PMID: 40090670 Free PMC article.
-
Comparison of pathologic response and survival outcomes between neoadjuvant chemoradiotherapy (nCRT) and neoadjuvant immunochemotherapy (nICT) in patients with locally advanced esophageal squamous cell carcinoma: a propensity score-matched analysis.BMC Cancer. 2024 Oct 5;24(1):1228. doi: 10.1186/s12885-024-12946-8. BMC Cancer. 2024. PMID: 39369225 Free PMC article.
-
Mid-term follow-up results of neoadjuvant sintilimab combined with chemotherapy for locally advanced resectable esophageal squamous cell carcinoma.Front Immunol. 2024 Dec 4;15:1453176. doi: 10.3389/fimmu.2024.1453176. eCollection 2024. Front Immunol. 2024. PMID: 39697347 Free PMC article.
-
Artificial intelligence in medical imaging empowers precision neoadjuvant immunochemotherapy in esophageal squamous cell carcinoma.J Immunother Cancer. 2025 Sep 9;13(9):e012468. doi: 10.1136/jitc-2025-012468. J Immunother Cancer. 2025. PMID: 40930744 Free PMC article. Review.
Cited by
-
CT-based delta-radiomics for predicting pathological response to neoadjuvant immunochemotherapy in esophageal squamous cell carcinoma: a multicenter study.BMC Med Imaging. 2024 Dec 3;24(1):329. doi: 10.1186/s12880-024-01503-1. BMC Med Imaging. 2024. PMID: 39627736 Free PMC article.
-
Gastrointestinal tumor personalized immunotherapy: an integrated analysis from molecular genetics to imaging biomarkers.Therap Adv Gastroenterol. 2025 Apr 23;18:17562848251333527. doi: 10.1177/17562848251333527. eCollection 2025. Therap Adv Gastroenterol. 2025. PMID: 40297204 Free PMC article. Review.
-
Sub-regional radiomics combining multichannel 2-dimensional or 3-dimensional deep learning for predicting neoadjuvant chemo-immunotherapy response in esophageal squamous cell carcinoma: a multicenter study.NPJ Precis Oncol. 2025 Jul 21;9(1):248. doi: 10.1038/s41698-025-01047-9. NPJ Precis Oncol. 2025. PMID: 40691312 Free PMC article.
-
Intestinal and esophageal microbiota in esophageal cancer development and treatment.Gut Microbes. 2025 Dec;17(1):2505118. doi: 10.1080/19490976.2025.2505118. Epub 2025 May 16. Gut Microbes. 2025. PMID: 40376843 Free PMC article. Review.
-
Photon-counting computed tomography in esophageal cancer: correlation of iodine concentration with histopathology and treatment response to neoadjuvant radiochemotherapy.Eur Radiol. 2025 May 23. doi: 10.1007/s00330-025-11683-1. Online ahead of print. Eur Radiol. 2025. PMID: 40410333
References
-
- Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4. - DOI - PubMed
-
- Luo H, Lu J, Bai Y, Mao T, Wang J, Fan Q, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–925. doi: 10.1001/jama.2021.12836. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

