YAP/TAZ activation mediates PQ-induced lung fibrosis by sustaining senescent pulmonary epithelial cells
- PMID: 38762455
- PMCID: PMC11102259
- DOI: 10.1186/s12931-024-02832-z
YAP/TAZ activation mediates PQ-induced lung fibrosis by sustaining senescent pulmonary epithelial cells
Abstract
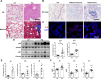
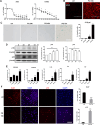
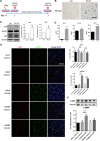
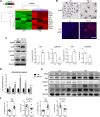
Paraquat (PQ) is a widely used herbicide and a common cause of poisoning that leads to pulmonary fibrosis with a high mortality rate. However, the underlying mechanisms of PQ-induced pulmonary fibrosis and whether pulmonary epithelial cell senescence is involved in the process remain elusive. In this study, PQ-induced pulmonary epithelial cell senescence and Hippo-YAP/TAZ activation were observed in both C57BL/6 mice and human epithelial cells. PQ-induced senescent pulmonary epithelial cells promoted lung fibroblast transformation through secreting senescence-associated secretory phenotype (SASP) factors. Yap/Taz knockdown in mice lungs significantly decreased the expression of downstream profibrotic protein Ctgf and senescent markers p16 and p21, and alleviated PQ-induced pulmonary fibrosis. Interfering YAP/TAZ in senescent human pulmonary epithelial cells resulted in decreased expression of the anti-apoptosis protein survivin and elevated level of apoptosis. In conclusion, our findings reveal a novel mechanism by which the involvement of Hippo-YAP/TAZ activation in pulmonary epithelial cell senescence mediates the pathogenesis of PQ-induced pulmonary fibrosis, thereby offering novel insights and potential targets for the clinical management of PQ poisoning as well as providing the mechanistic insight of the involvement of Yap/Taz activation in cell senescence in pulmonary fibrosis and its related pulmonary disorders. The YIN YANG balance between cell senescence and apoptosis is important to maintain the homeostasis of the lung, the disruption of which will lead to disease.
Keywords: Cellular senescence; Paraquat; Poisoning; Pulmonary epithelial cell; Pulmonary fibrosis; YAP/TAZ.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
YAP/TAZ are crucial regulators of macrophage-mediated pulmonary inflammation and fibrosis after bleomycin-induced injury.Eur Respir J. 2025 Jun 5;65(6):2301544. doi: 10.1183/13993003.01544-2023. Print 2025 Jun. Eur Respir J. 2025. PMID: 39915054 Free PMC article.
-
YAP/TAZ Signaling in the Pathobiology of Pulmonary Fibrosis.Cells. 2024 Sep 10;13(18):1519. doi: 10.3390/cells13181519. Cells. 2024. PMID: 39329703 Free PMC article. Review.
-
Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis.Am J Physiol Lung Cell Mol Physiol. 2015 Feb 15;308(4):L344-57. doi: 10.1152/ajplung.00300.2014. Epub 2014 Dec 12. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 25502501 Free PMC article. Clinical Trial.
-
YAP/TAZ-mediated nuclear membrane rupture in promoting senescence of skeletal muscle associated with COPD.Respir Res. 2025 Mar 12;26(1):98. doi: 10.1186/s12931-025-03170-4. Respir Res. 2025. PMID: 40075503 Free PMC article.
-
Role of the Hippo-YAP/TAZ Pathway in Epithelioid Hemangioendothelioma and its Potential as a Therapeutic Target.Anticancer Res. 2024 Oct;44(10):4147-4153. doi: 10.21873/anticanres.17245. Anticancer Res. 2024. PMID: 39348982 Review.
References
-
- Chinta SJ, Woods G, Demaria M, Rane A, Zou Y, McQuade A, Rajagopalan S, Limbad C, Madden DT, Campisi J, Andersen JK. Cellular Senescence is Induced by the Environmental Neurotoxin Paraquat and contributes to Neuropathology Linked to Parkinson’s Disease. Cell Rep. 2018;22(4):930–40. doi: 10.1016/j.celrep.2017.12.092. - DOI - PMC - PubMed
-
- Liu XB, Yang H, Liu ZN. Signaling pathways involved in paraquat-induced pulmonary toxicity: Molecular mechanisms and potential therapeutic drugs. Int Immunopharmacol 2022, 113. - PubMed
-
- Yin Y, Guo X, Zhang SL, Sun CY. Analysis of Paraquat Intoxication Epidemic (2002–2011) within China. Biomed Environ Sci. 2013;26(6):509–12. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

