Regulatory mechanisms of PD-1/PD-L1 in cancers
- PMID: 38762484
- PMCID: PMC11102195
- DOI: 10.1186/s12943-024-02023-w
Regulatory mechanisms of PD-1/PD-L1 in cancers
Abstract
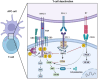
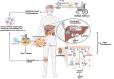
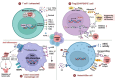
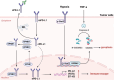
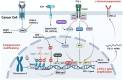
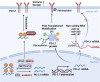
Immune evasion contributes to cancer growth and progression. Cancer cells have the ability to activate different immune checkpoint pathways that harbor immunosuppressive functions. The programmed death protein 1 (PD-1) and programmed cell death ligands (PD-Ls) are considered to be the major immune checkpoint molecules. The interaction of PD-1 and PD-L1 negatively regulates adaptive immune response mainly by inhibiting the activity of effector T cells while enhancing the function of immunosuppressive regulatory T cells (Tregs), largely contributing to the maintenance of immune homeostasis that prevents dysregulated immunity and harmful immune responses. However, cancer cells exploit the PD-1/PD-L1 axis to cause immune escape in cancer development and progression. Blockade of PD-1/PD-L1 by neutralizing antibodies restores T cells activity and enhances anti-tumor immunity, achieving remarkable success in cancer therapy. Therefore, the regulatory mechanisms of PD-1/PD-L1 in cancers have attracted an increasing attention. This article aims to provide a comprehensive review of the roles of the PD-1/PD-L1 signaling in human autoimmune diseases and cancers. We summarize all aspects of regulatory mechanisms underlying the expression and activity of PD-1 and PD-L1 in cancers, including genetic, epigenetic, post-transcriptional and post-translational regulatory mechanisms. In addition, we further summarize the progress in clinical research on the antitumor effects of targeting PD-1/PD-L1 antibodies alone and in combination with other therapeutic approaches, providing new strategies for finding new tumor markers and developing combined therapeutic approaches.
Keywords: Combination therapy; PD-1; PD-L1; Regulatory mechanism; Tumor immunity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Programmed death receptor (PD-)1/PD-ligand (L)1 in urological cancers : the "all-around warrior" in immunotherapy.Mol Cancer. 2024 Sep 2;23(1):183. doi: 10.1186/s12943-024-02095-8. Mol Cancer. 2024. PMID: 39223527 Free PMC article. Review.
-
Augmenting Anticancer Immunity Through Combined Targeting of Angiogenic and PD-1/PD-L1 Pathways: Challenges and Opportunities.Front Immunol. 2020 Nov 5;11:598877. doi: 10.3389/fimmu.2020.598877. eCollection 2020. Front Immunol. 2020. PMID: 33250900 Free PMC article. Review.
-
Fructose-1,6-bisphosphatase loss modulates STAT3-dependent expression of PD-L1 and cancer immunity.Theranostics. 2020 Jan 1;10(3):1033-1045. doi: 10.7150/thno.38137. eCollection 2020. Theranostics. 2020. PMID: 31938049 Free PMC article.
-
The Extrinsic and Intrinsic Roles of PD-L1 and Its Receptor PD-1: Implications for Immunotherapy Treatment.Front Immunol. 2020 Oct 21;11:568931. doi: 10.3389/fimmu.2020.568931. eCollection 2020. Front Immunol. 2020. PMID: 33193345 Free PMC article. Review.
-
Cross-Talks between Raf Kinase Inhibitor Protein and Programmed Cell Death Ligand 1 Expressions in Cancer: Role in Immune Evasion and Therapeutic Implications.Cells. 2024 May 17;13(10):864. doi: 10.3390/cells13100864. Cells. 2024. PMID: 38786085 Free PMC article. Review.
Cited by
-
Hepatotoxicity in Cancer Immunotherapy: Diagnosis, Management, and Future Perspectives.Cancers (Basel). 2024 Dec 29;17(1):76. doi: 10.3390/cancers17010076. Cancers (Basel). 2024. PMID: 39796705 Free PMC article. Review.
-
The Role of SWI/SNF Complex in Bladder Cancer.J Cell Mol Med. 2025 Jan;29(1):e70348. doi: 10.1111/jcmm.70348. J Cell Mol Med. 2025. PMID: 39779467 Free PMC article. Review.
-
Unraveling the triad of immunotherapy, tumor microenvironment, and skeletal muscle biomechanics in oncology.Front Immunol. 2025 Apr 2;16:1572821. doi: 10.3389/fimmu.2025.1572821. eCollection 2025. Front Immunol. 2025. PMID: 40242775 Free PMC article. Review.
-
Targeted Delivery of BMS-1166 for Enhanced Breast Cancer Immunotherapy.Int J Nanomedicine. 2025 Jan 8;20:293-308. doi: 10.2147/IJN.S497089. eCollection 2025. Int J Nanomedicine. 2025. PMID: 39802387 Free PMC article.
-
Comprehensive overview of antibody drug-related clinical studies in gynecology: insights from ClinicalTrials.gov.Front Med (Lausanne). 2025 May 9;12:1521587. doi: 10.3389/fmed.2025.1521587. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40417700 Free PMC article.
References
-
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol Baltim Md. 1950. 2002;169:5538–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1053320221155/the Independent Exploration and Innovation Program of Central South University
- U23A20456, 82272631, 82072596, 82173339/The National Natural Science Foundation of China
- #111-2-12/the National "111" Project
- 2022SK2026/the Hunan Provincial Key Research and Development Program
- 2023SK2094/the scientific research program of FuRong laboratory
LinkOut - more resources
Full Text Sources
Medical
Research Materials

