Hsc70 promotes anti-tumor immunity by targeting PD-L1 for lysosomal degradation
- PMID: 38762492
- PMCID: PMC11102475
- DOI: 10.1038/s41467-024-48597-3
Hsc70 promotes anti-tumor immunity by targeting PD-L1 for lysosomal degradation
Abstract
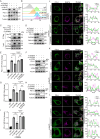
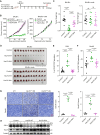
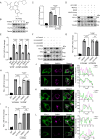
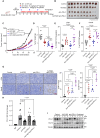
Immune checkpoint inhibition targeting the PD-1/PD-L1 pathway has become a powerful clinical strategy for treating cancer, but its efficacy is complicated by various resistance mechanisms. One of the reasons for the resistance is the internalization and recycling of PD-L1 itself upon antibody binding. The inhibition of lysosome-mediated degradation of PD-L1 is critical for preserving the amount of PD-L1 recycling back to the cell membrane. In this study, we find that Hsc70 promotes PD-L1 degradation through the endosome-lysosome pathway and reduces PD-L1 recycling to the cell membrane. This effect is dependent on Hsc70-PD-L1 binding which inhibits the CMTM6-PD-L1 interaction. We further identify an Hsp90α/β inhibitor, AUY-922, which induces Hsc70 expression and PD-L1 lysosomal degradation. Either Hsc70 overexpression or AUY-922 treatment can reduce PD-L1 expression, inhibit tumor growth and promote anti-tumor immunity in female mice; AUY-922 can further enhance the anti-tumor efficacy of anti-PD-L1 and anti-CTLA4 treatment. Our study elucidates a molecular mechanism of Hsc70-mediated PD-L1 lysosomal degradation and provides a target and therapeutic strategies for tumor immunotherapy.
© 2024. The Author(s).
Conflict of interest statement
X.X., H.X., T.X., M.Z., Y.X. and Z.C. have filed a patent application “the use of drug combinations, 202311744204.1”. The remaining authors declare no other competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

