Stimulation of tumoricidal immunity via bacteriotherapy inhibits glioblastoma relapse
- PMID: 38762500
- PMCID: PMC11102507
- DOI: 10.1038/s41467-024-48606-5
Stimulation of tumoricidal immunity via bacteriotherapy inhibits glioblastoma relapse
Abstract
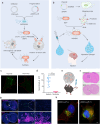
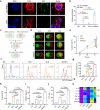
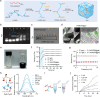
Glioblastoma multiforme (GBM) is a highly aggressive brain tumor characterized by invasive behavior and a compromised immune response, presenting treatment challenges. Surgical debulking of GBM fails to address its highly infiltrative nature, leaving neoplastic satellites in an environment characterized by impaired immune surveillance, ultimately paving the way for tumor recurrence. Tracking and eradicating residual GBM cells by boosting antitumor immunity is critical for preventing postoperative relapse, but effective immunotherapeutic strategies remain elusive. Here, we report a cavity-injectable bacterium-hydrogel superstructure that targets GBM satellites around the cavity, triggers GBM pyroptosis, and initiates innate and adaptive immune responses, which prevent postoperative GBM relapse in male mice. The immunostimulatory Salmonella delivery vehicles (SDVs) engineered from attenuated Salmonella typhimurium (VNP20009) seek and attack GBM cells. Salmonella lysis-inducing nanocapsules (SLINs), designed to trigger autolysis, are tethered to the SDVs, eliciting antitumor immune response through the intracellular release of bacterial components. Furthermore, SDVs and SLINs administration via intracavitary injection of the ATP-responsive hydrogel can recruit phagocytes and promote antigen presentation, initiating an adaptive immune response. Therefore, our work offers a local bacteriotherapy for stimulating anti-GBM immunity, with potential applicability for patients facing malignancies at a high risk of recurrence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy.Sci Transl Med. 2022 Aug 3;14(656):eabn1128. doi: 10.1126/scitranslmed.abn1128. Epub 2022 Aug 3. Sci Transl Med. 2022. PMID: 35921473
-
Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection.Nat Nanotechnol. 2021 May;16(5):538-548. doi: 10.1038/s41565-020-00843-7. Epub 2021 Feb 1. Nat Nanotechnol. 2021. PMID: 33526838
-
Immunostimulatory silica nanoparticle boosts innate immunity in brain tumors.Nanoscale Horiz. 2021 Feb 1;6(2):156-167. doi: 10.1039/d0nh00446d. Epub 2021 Jan 5. Nanoscale Horiz. 2021. PMID: 33400743 Free PMC article.
-
Current progress in chimeric antigen receptor T cell therapy for glioblastoma multiforme.Cancer Med. 2021 Aug;10(15):5019-5030. doi: 10.1002/cam4.4064. Epub 2021 Jun 19. Cancer Med. 2021. PMID: 34145792 Free PMC article. Review.
-
The CNS and the Brain Tumor Microenvironment: Implications for Glioblastoma Immunotherapy.Int J Mol Sci. 2020 Oct 5;21(19):7358. doi: 10.3390/ijms21197358. Int J Mol Sci. 2020. PMID: 33027976 Free PMC article. Review.
Cited by
-
Interactions between tumor-associated macrophages and regulated cell death: therapeutic implications in immuno-oncology.Front Oncol. 2024 Nov 7;14:1449696. doi: 10.3389/fonc.2024.1449696. eCollection 2024. Front Oncol. 2024. PMID: 39575419 Free PMC article. Review.
-
Aggregation induced emission luminogen bacteria hybrid bionic robot for multimodal phototheranostics and immunotherapy.Nat Commun. 2025 Mar 16;16(1):2578. doi: 10.1038/s41467-025-57533-y. Nat Commun. 2025. PMID: 40089477 Free PMC article.
-
Genetically-engineered Salmonella typhimurium expressing FGF21 promotes neurological recovery in ischemic stroke via FGFR1/AMPK/mTOR pathway.J Neuroinflammation. 2025 Jun 28;22(1):170. doi: 10.1186/s12974-025-03498-0. J Neuroinflammation. 2025. PMID: 40581646 Free PMC article.
-
Enhanced antitumor immunity of VNP20009-CCL2-CXCL9 via the cGAS/STING axis in osteosarcoma lung metastasis.J Immunother Cancer. 2025 Jul 1;13(7):e012269. doi: 10.1136/jitc-2025-012269. J Immunother Cancer. 2025. PMID: 40592739 Free PMC article.
-
γ-Glutamyl transpeptidase-activable nanoprobe crosses the blood-brain barrier for immuno-sonodynamic therapy of glioma.Nat Commun. 2024 Nov 29;15(1):10418. doi: 10.1038/s41467-024-54382-z. Nat Commun. 2024. PMID: 39613729 Free PMC article.
References
MeSH terms
Grants and funding
- 82303810/National Natural Science Foundation of China (National Science Foundation of China)
- 82111530202/National Natural Science Foundation of China (National Science Foundation of China)
- 82172740/National Natural Science Foundation of China (National Science Foundation of China)
- ZR2023QH224/Natural Science Foundation of Shandong Province (Shandong Provincial Natural Science Foundation)
- ZR2022ZD17/Natural Science Foundation of Shandong Province (Shandong Provincial Natural Science Foundation)
LinkOut - more resources
Full Text Sources
Medical

