AMPK-a key factor in crosstalk between tumor cell energy metabolism and immune microenvironment?
- PMID: 38762523
- PMCID: PMC11102436
- DOI: 10.1038/s41420-024-02011-5
AMPK-a key factor in crosstalk between tumor cell energy metabolism and immune microenvironment?
Abstract
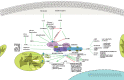
Immunotherapy has now garnered significant attention as an essential component in cancer therapy during this new era. However, due to immune tolerance, immunosuppressive environment, tumor heterogeneity, immune escape, and other factors, the efficacy of tumor immunotherapy has been limited with its application to very small population size. Energy metabolism not only affects tumor progression but also plays a crucial role in immune escape. Tumor cells are more metabolically active and need more energy and nutrients to maintain their growth, which causes the surrounding immune cells to lack glucose, oxygen, and other nutrients, with the result of decreased immune cell activity and increased immunosuppressive cells. On the other hand, immune cells need to utilize multiple metabolic pathways, for instance, cellular respiration, and oxidative phosphorylation pathways to maintain their activity and normal function. Studies have shown that there is a significant difference in the energy expenditure of immune cells in the resting and activated states. Notably, competitive uptake of glucose is the main cause of impaired T cell function. Conversely, glutamine competition often affects the activation of most immune cells and the transformation of CD4+T cells into inflammatory subtypes. Excessive metabolite lactate often impairs the function of NK cells. Furthermore, the metabolite PGE2 also often inhibits the immune response by inhibiting Th1 differentiation, B cell function, and T cell activation. Additionally, the transformation of tumor-suppressive M1 macrophages into cancer-promoting M2 macrophages is influenced by energy metabolism. Therefore, energy metabolism is a vital factor and component involved in the reconstruction of the tumor immune microenvironment. Noteworthy and vital is that not only does the metabolic program of tumor cells affect the antigen presentation and recognition of immune cells, but also the metabolic program of immune cells affects their own functions, ultimately leading to changes in tumor immune function. Metabolic intervention can not only improve the response of immune cells to tumors, but also increase the immunogenicity of tumors, thereby expanding the population who benefit from immunotherapy. Consequently, identifying metabolic crosstalk molecules that link tumor energy metabolism and immune microenvironment would be a promising anti-tumor immune strategy. AMPK (AMP-activated protein kinase) is a ubiquitous serine/threonine kinase in eukaryotes, serving as the central regulator of metabolic pathways. The sequential activation of AMPK and its associated signaling cascades profoundly impacts the dynamic alterations in tumor cell bioenergetics. By modulating energy metabolism and inflammatory responses, AMPK exerts significant influence on tumor cell development, while also playing a pivotal role in tumor immunotherapy by regulating immune cell activity and function. Furthermore, AMPK-mediated inflammatory response facilitates the recruitment of immune cells to the tumor microenvironment (TIME), thereby impeding tumorigenesis, progression, and metastasis. AMPK, as the link between cell energy homeostasis, tumor bioenergetics, and anti-tumor immunity, will have a significant impact on the treatment and management of oncology patients. That being summarized, the main objective of this review is to pinpoint the efficacy of tumor immunotherapy by regulating the energy metabolism of the tumor immune microenvironment and to provide guidance for the development of new immunotherapy strategies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment.Front Immunol. 2023 Feb 15;14:1114582. doi: 10.3389/fimmu.2023.1114582. eCollection 2023. Front Immunol. 2023. PMID: 36875093 Free PMC article. Review.
-
Immune-mediated anti-tumor effects of metformin; targeting metabolic reprogramming of T cells as a new possible mechanism for anti-cancer effects of metformin.Biochem Pharmacol. 2020 Apr;174:113787. doi: 10.1016/j.bcp.2019.113787. Epub 2019 Dec 27. Biochem Pharmacol. 2020. PMID: 31884044 Review.
-
A novel strategy to fuel cancer immunotherapy: targeting glucose metabolism to remodel the tumor microenvironment.Front Oncol. 2022 Jul 18;12:931104. doi: 10.3389/fonc.2022.931104. eCollection 2022. Front Oncol. 2022. PMID: 35924168 Free PMC article. Review.
-
Manipulating T-cell metabolism to enhance immunotherapy in solid tumor.Front Immunol. 2022 Dec 22;13:1090429. doi: 10.3389/fimmu.2022.1090429. eCollection 2022. Front Immunol. 2022. PMID: 36618408 Free PMC article. Review.
-
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.Cell Signal. 2013 Oct;25(10):1939-48. doi: 10.1016/j.cellsig.2013.06.007. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770291 Review.
Cited by
-
Hyaluronic acid-coated polypeptide nanogel enhances specific distribution and therapy of tacrolimus in rheumatoid arthritis.J Nanobiotechnology. 2024 Sep 6;22(1):547. doi: 10.1186/s12951-024-02784-y. J Nanobiotechnology. 2024. PMID: 39238027 Free PMC article.
-
Protective Role of Oxycodone in Myocardial Oxidative Stress and Mitochondrial Dysfunction Induced by Ischemia-Reperfusion.J Biochem Mol Toxicol. 2025 Feb;39(2):e70151. doi: 10.1002/jbt.70151. J Biochem Mol Toxicol. 2025. PMID: 39865943 Free PMC article.
-
Sympathetic nervous system in tumor progression and metabolic regulation: mechanisms and clinical potential.J Transl Med. 2025 Jul 25;23(1):836. doi: 10.1186/s12967-025-06657-2. J Transl Med. 2025. PMID: 40713825 Free PMC article. Review.
-
Identification of a novel immune-related gene signature by single-cell and bulk sequencing for the prediction of the immune landscape and prognosis of breast cancer.Cancer Cell Int. 2024 Dec 3;24(1):393. doi: 10.1186/s12935-024-03589-7. Cancer Cell Int. 2024. PMID: 39627792 Free PMC article.
-
The Complexity of Malignant Glioma Treatment.Cancers (Basel). 2025 Mar 4;17(5):879. doi: 10.3390/cancers17050879. Cancers (Basel). 2025. PMID: 40075726 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA: A Cancer J Clin. 2022;72:7–33. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

