The circulating proteome and brain health: Mendelian randomisation and cross-sectional analyses
- PMID: 38762535
- PMCID: PMC11102511
- DOI: 10.1038/s41398-024-02915-x
The circulating proteome and brain health: Mendelian randomisation and cross-sectional analyses
Abstract
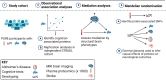
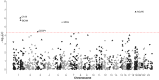
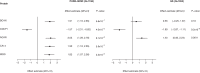
Decline in cognitive function is the most feared aspect of ageing. Poorer midlife cognitive function is associated with increased dementia and stroke risk. The mechanisms underlying variation in cognitive function are uncertain. Here, we assessed associations between 1160 proteins' plasma levels and two measures of cognitive function, the digit symbol substitution test (DSST) and the Montreal Cognitive Assessment in 1198 PURE-MIND participants. We identified five DSST performance-associated proteins (NCAN, BCAN, CA14, MOG, CDCP1), with NCAN and CDCP1 showing replicated association in an independent cohort, GS (N = 1053). MRI-assessed structural brain phenotypes partially mediated (8-19%) associations between NCAN, BCAN, and MOG, and DSST performance. Mendelian randomisation analyses suggested higher CA14 levels might cause larger hippocampal volume and increased stroke risk, whilst higher CDCP1 levels might increase intracranial aneurysm risk. Our findings highlight candidates for further study and the potential for drug repurposing to reduce the risk of stroke and cognitive decline.
© 2024. The Author(s).
Conflict of interest statement
MC is supported by a Canadian Institute of Health Research doctoral award and has received consulting fees from Bayer AG. MP is supported by the EJ Moran Campbell Internal Career Research Award from McMaster University. DAG is a part-time employee of Optima partners, a health data consultancy based at the Bayes centre, The University of Edinburgh. SH is an employee of Bayer AG. AMM has previously received speaker’s fees from Illumina and Janssen and research grant funding from The Sackler Trust. SY is supported by the Heart and Stroke Foundation/Marion W Burke Chair in Cardiovascular Disease. GP is supported by the CISCO Professorship in Integrated Health Systems. The other authors declare no competing interests.
Figures

References
-
- Martin GM. Defeating Dementia. Nature. 2004;431:247–8. doi: 10.1038/431247b. - DOI
MeSH terms
Grants and funding
- MC_PC_17215/RCUK | Medical Research Council (MRC)
- 399497/Heart and Stroke Foundation of Canada (Heart and Stroke Foundation)
- 220857/Z/20/Z/Wellcome Trust (Wellcome)
- 204804/Z/16/Z/Wellcome Trust (Wellcome)
- MC_PC_17209/RCUK | Medical Research Council (MRC)
- MR/L023784/2/RCUK | Medical Research Council (MRC)
- G-18-0022359/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- 108890/Z/15/Z/Wellcome Trust (Wellcome)
- 104036/Z/14/Z/Wellcome Trust (Wellcome)
- WT_/Wellcome Trust/United Kingdom
- 216767/Z/19/Z/Wellcome Trust (Wellcome)
- 173096/Lister Institute of Preventive Medicine
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

