Monitoring monocyte HLA-DR expression and CD4 + T lymphocyte count in dexamethasone-treated severe COVID-19 patients
- PMID: 38762684
- PMCID: PMC11102415
- DOI: 10.1186/s13613-024-01310-5
Monitoring monocyte HLA-DR expression and CD4 + T lymphocyte count in dexamethasone-treated severe COVID-19 patients
Abstract
Background: A 10-day dexamethasone regimen has emerged as the internationally adopted standard-of-care for severe COVID-19 patients. However, the immune response triggered by SARS-CoV-2 infection remains a complex and dynamic phenomenon, leading to various immune profiles and trajectories. The immune status of severe COVID-19 patients following complete dexamethasone treatment has yet to be thoroughly documented.
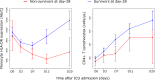
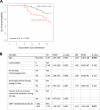
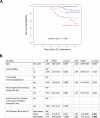
Results: To analyze monocyte HLA-DR expression (mHLA-DR) and CD4 + T lymphocyte count (CD4) in critically ill COVID-19 patients after a dexamethasone course and evaluate their association with 28-day ICU mortality, adult COVID-19 patients (n = 176) with an ICU length of stay of at least 10 days and under dexamethasone treatment were included. Associations between each biomarker value (or in combination) measured at day 10 after ICU admission and 28-day mortality in ICU were evaluated. At day 10, the majority of patients presented decreased values of both parameters. A significant association between low mHLA-DR and 28-day mortality was observed. This association remained significant in a multivariate analysis including age, comorbidities or pre-existing immunosuppression (adjusted Hazard ratio (aHR) = 2.86 [1.30-6.32], p = 0.009). Similar results were obtained with decreased CD4 + T cell count (aHR = 2.10 [1.09-4.04], p = 0.027). When combining these biomarkers, patients with both decreased mHLA-DR and low CD4 presented with an independent and significant elevated risk of 28-day mortality (i.e., 60%, aHR = 4.83 (1.72-13.57), p = 0.001).
Conclusions: By using standardized immunomonitoring tools available in clinical practice, it is possible to identify a subgroup of patients at high risk of mortality at the end of a 10-day dexamethasone treatment. This emphasizes the significance of integrating immune monitoring into the surveillance of intensive care patients in order to guide further immumodulation approaches.
Keywords: CD4; COVID-19; Dexamethasone; HLA-DR; Immunomonitoring; Monocyte; Sepsis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing financial interests in relation to the work.
Figures


References
-
- Lamontagne F, Agarwal A, Rochwerg B, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for COVID-19. BMJ. 2020;370:m3379. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

