Drug release profile of a novel exenatide long-term drug delivery system (OKV-119) administered to cats
- PMID: 38762728
- PMCID: PMC11102179
- DOI: 10.1186/s12917-024-04051-6
Drug release profile of a novel exenatide long-term drug delivery system (OKV-119) administered to cats
Abstract
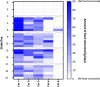
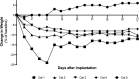
Beneficial weight-loss properties of glucagon-like peptide-1 receptor agonists (GLP-1RA) in obese people, with corresponding improvements in cardiometabolic risk factors, are well established. OKV-119 is an investigational drug delivery system that is being developed for the long-term delivery of the GLP-1RA exenatide to feline patients. The purpose of this study was to evaluate the drug release characteristics of subcutaneous OKV-119 implants configured to release exenatide for 84 days. Following a 7-day acclimation period, five purpose-bred cats were implanted with OKV-119 protypes and observed for a 112-day study period. Food intake, weekly plasma exenatide concentrations and body weight were measured. Exenatide plasma concentrations were detected at the first measured timepoint (Day 7) and maintained above baseline for over 84 Days. Over the first 28 days, reduced caloric intake and a reduction in body weight were observed in four of five cats. In these cats, a body weight reduction of at least 5% was maintained throughout the 112-day study period. This study demonstrates that a single OKV-119 implant can deliver the GLP-1RA exenatide for a months long duration. Results suggest that exposure to exenatide plasma concentrations ranging from 1.5 ng/ml to 4 ng/ml are sufficient for inducing weight loss in cats.
Keywords: Adherence; Drug delivery; Exenatide; Feline diabetes; Feline obesity.
© 2024. The Author(s).
Conflict of interest statement
MK and WHA are Okava Pharmaceuticals shareholders. CG is a consultant to Okava Pharmaceuticals.
Figures

References
-
- Organization WH. Obesity and overweight. Fact Sheet 2021; https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
-
- Federation WO. World Obesity Atlas. 2022.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

