Stacked probability plots of the extended illness-death model using constant transition hazards - an easy to use shiny app
- PMID: 38762731
- PMCID: PMC11102298
- DOI: 10.1186/s12874-024-02240-3
Stacked probability plots of the extended illness-death model using constant transition hazards - an easy to use shiny app
Abstract
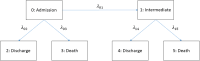
Background: Extended illness-death models (a specific class of multistate models) are a useful tool to analyse situations like hospital-acquired infections, ventilation-associated pneumonia, and transfers between hospitals. The main components of these models are hazard rates and transition probabilities. Calculation of different measures and their interpretation can be challenging due to their complexity.
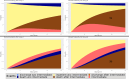
Methods: By assuming time-constant hazards, the complexity of these models becomes manageable and closed mathematical forms for transition probabilities can be derived. Using these forms, we created a tool in R to visualize transition probabilities via stacked probability plots.
Results: In this article, we present this tool and give some insights into its theoretical background. Using published examples, we give guidelines on how this tool can be used. Our goal is to provide an instrument that helps obtain a deeper understanding of a complex multistate setting.
Conclusion: While multistate models (in particular extended illness-death models), can be highly complex, this tool can be used in studies to both understand assumptions, which have been made during planning and as a first step in analysing complex data structures. An online version of this tool can be found at https://eidm.imbi.uni-freiburg.de/ .
Keywords: Extended illness-death model; Hazard rates; Markov process; R; Shiny app; Stacked probability plot; Transition probability.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- European Medicines Agency. ICH E9 (R1.) addendum on estimands and sensitivity analysis in clinical trials to the guideline on statistical principles for clinical trials [Internet]. Verfügbar unter: https://www.ema.europa.eu/en/ich-e9-statistical-principles-clinical-tria....
-
- Erdmann A, Beyersmann J, Rufibach K. Oncology clinical trial design planning based on a multistate model that jointly models progression-free and overall survival endpoints. 2023 [zitiert 13. Februar 2024]; Verfügbar unter: https://arxiv.org/abs/2301.10059. - PMC - PubMed
-
- Wolkewitz M, Cooper BS, Bonten MJM, Barnett AG, Schumacher M. Interpreting and comparing risks in the presence of competing events. BMJ 21 August. 2014;349(aug21 5):g5060–5060. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources