Percutaneous bone marrow concentrate and platelet products versus exercise therapy for the treatment of rotator cuff tears: a randomized controlled, crossover trial with 2-year follow-up
- PMID: 38762734
- PMCID: PMC11102209
- DOI: 10.1186/s12891-024-07519-6
Percutaneous bone marrow concentrate and platelet products versus exercise therapy for the treatment of rotator cuff tears: a randomized controlled, crossover trial with 2-year follow-up
Abstract
Background: Surgical repair is recommended for the treatment of high-grade partial and full thickness rotator cuff tears, although evidence shows surgery is not necessarily superior to non-surgical therapy. The purpose of this study was to compare percutaneous orthobiologic treatment to a home exercise therapy program for supraspinatus tears.
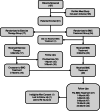
Methods: In this randomized-controlled, crossover design, participants with a torn supraspinatus tendon received either 'BMC treatment', consisting of a combination of autologous bone marrow concentrate (BMC) and platelet products, or underwent a home exercise therapy program. After three months, patients randomized to exercise therapy could crossover to receive BMC treatment if not satisfied with shoulder progression. Patient-reported outcomes of Numeric Pain Scale (NPS), Disabilities of the Arm, Shoulder, and Hand, (DASH), and a modified Single Assessment Numeric Evaluation (SANE) were collected at 1, 3, 6, 12, and 24 months. Pre- and post-treatment MRI were assessed using the Snyder Classification system.
Results: Fifty-one patients were enrolled and randomized to the BMC treatment group (n = 34) or the exercise therapy group (n = 17). Significantly greater improvement in median ΔDASH, ΔNPS, and SANE scores were reported by the BMC treatment group compared to the exercise therapy group (-11.7 vs -3.8, P = 0.01; -2.0 vs 0.5, P = 0.004; and 50.0 vs 0.0, P < 0.001; respectively) after three months. Patient-reported outcomes continued to progress through the study's two-year follow-up period without a serious adverse event. Of patients with both pre- and post-treatment MRIs, a majority (73%) showed evidence of healing post-BMC treatment.
Conclusions: Patients reported significantly greater changes in function, pain, and overall improvement following BMC treatment compared to exercise therapy for high grade partial and full thickness supraspinatus tears.
Trial registration: This protocol was registered with www.
Clinicaltrials: gov (NCT01788683; 11/02/2013).
Keywords: Autologous orthobiologics; Bone marrow concentrate (BMC); Cell therapy; Exercise therapy; Musculoskeletal pain; Platelet-rich plasma (PRP); Rotator cuff tears; Shoulder.
© 2024. The Author(s).
Conflict of interest statement
One or more of the authors has declared a potential conflict of interest. ZF, ED, DRB, and NJS are or were previously employed by Regenexx, LLC. CJC is a shareholder, patent holder, and Chief Medical Officer of Regenexx, LLC and an owner of the Centeno-Schultz Clinic.
Figures





Similar articles
-
A Randomized Controlled Trial of the Treatment of Rotator Cuff Tears with Bone Marrow Concentrate and Platelet Products Compared to Exercise Therapy: A Midterm Analysis.Stem Cells Int. 2020 Jan 30;2020:5962354. doi: 10.1155/2020/5962354. eCollection 2020. Stem Cells Int. 2020. PMID: 32399045 Free PMC article.
-
Surgery for rotator cuff tears.Cochrane Database Syst Rev. 2019 Dec 9;12(12):CD013502. doi: 10.1002/14651858.CD013502. Cochrane Database Syst Rev. 2019. PMID: 31813166 Free PMC article.
-
Prospective Randomized Trial of Biologic Augmentation With Bone Marrow Aspirate Concentrate in Patients Undergoing Arthroscopic Rotator Cuff Repair.Am J Sports Med. 2023 Apr;51(5):1234-1242. doi: 10.1177/03635465231154601. Epub 2023 Feb 22. Am J Sports Med. 2023. PMID: 36811557 Clinical Trial.
-
A Midterm Evaluation of Postoperative Platelet-Rich Plasma Injections on Arthroscopic Supraspinatus Repair: A Randomized Controlled Trial.Am J Sports Med. 2017 Nov;45(13):2965-2974. doi: 10.1177/0363546517719048. Epub 2017 Aug 14. Am J Sports Med. 2017. PMID: 28806095 Clinical Trial.
-
Subacromial decompression surgery for rotator cuff disease.Cochrane Database Syst Rev. 2019 Jan 17;1(1):CD005619. doi: 10.1002/14651858.CD005619.pub3. Cochrane Database Syst Rev. 2019. PMID: 30707445 Free PMC article.
Cited by
-
Clinical outcomes of tibial nonunion treatment through the combination of PRP, autogenous iliac bone grafting, and augmentation plating.Front Surg. 2025 Jul 3;12:1573679. doi: 10.3389/fsurg.2025.1573679. eCollection 2025. Front Surg. 2025. PMID: 40677636 Free PMC article.
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

